Difference between revisions of "Formation water density"
Cwhitehurst (talk | contribs) |
Testaccount (talk | contribs) (Undo revision 28124 by Testaccount (talk)) |
||
| (15 intermediate revisions by 3 users not shown) | |||
| Line 6: | Line 6: | ||
| part = Critical elements of the petroleum system | | part = Critical elements of the petroleum system | ||
| chapter = Formation fluid pressure and its application | | chapter = Formation fluid pressure and its application | ||
| − | | frompg = 5- | + | | frompg = 5-16 |
| − | | topg = 5- | + | | topg = 5-18 |
| author = Edward A. Beaumont, Forrest Fiedler | | author = Edward A. Beaumont, Forrest Fiedler | ||
| link = http://archives.datapages.com/data/specpubs/beaumont/ch05/ch05.htm | | link = http://archives.datapages.com/data/specpubs/beaumont/ch05/ch05.htm | ||
| Line 20: | Line 20: | ||
* Total dissolved solids (TDS) | * Total dissolved solids (TDS) | ||
| − | It is the mass of the formation water per unit volume of the formation water and is given in metric units (g/cm<sup>3</sup>). For reservoir engineering calculations, it is considered equivalent to specific gravity. | + | It is the mass of the formation water per unit volume of the formation water and is given in metric units (g/cm<sup>3</sup>). For reservoir engineering calculations, it is considered equivalent to specific [[gravity]]. |
==Estimating density from TDS== | ==Estimating density from TDS== | ||
| Line 28: | Line 28: | ||
==Procedure: estimating density from r<sub>w</sub>== | ==Procedure: estimating density from r<sub>w</sub>== | ||
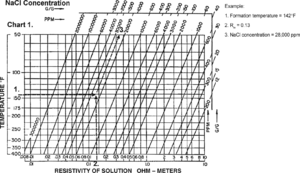
| + | [[file:formation-fluid-pressure-and-its-application_fig5-5.png|300px|thumb|{{figure number|1}}Determining NaCl concentration. Copyright: Schlumberger.]] | ||
| + | |||
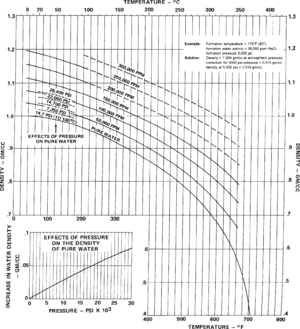
| + | [[file:formation-fluid-pressure-and-its-application_fig5-6.png|300px|thumb|{{figure number|2}}Use to estimate formation water density from ppm NaCl and temperature. Copyright: Gearhart-Owens.<ref name=G-O1972>Gearhart-Owens Industries, 1972, GO Log Interpretation Reference Data Handbook: Fort Worth, Gearhart-Owens Industries Inc., 226 p</ref>]] | ||
| + | |||
Use the procedure outlined in the table below to estimate formation water density at reservoir conditions using R<sub>w</sub>. The approximate error is ±10% (after <ref name=ch05r4 />). | Use the procedure outlined in the table below to estimate formation water density at reservoir conditions using R<sub>w</sub>. The approximate error is ±10% (after <ref name=ch05r4 />). | ||
| − | + | # Gather data: formation temperature (T<sub>f</sub> ), water resistivity (R<sub>w</sub> ), and formation pressure. Estimate pressure by multiplying depth by 0.433 psi/ft or other appropriate gradient. Check for T<sub>f</sub> errors. | |
| − | + | # Estimate sodium chloride (NaCl) concentration from R using [[:file:formation-fluid-pressure-and-its-application_fig5-5.png|Figure 1]]. | |
| − | + | # Estimate density from wt % NaCl and temperature using [[:file:formation-fluid-pressure-and-its-application_fig5-6.png|Figure 2]]. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
==Gather data== | ==Gather data== | ||
| Line 51: | Line 44: | ||
* Formation water resistivity | * Formation water resistivity | ||
| − | + | # Estimate formation temperature T<sub>f</sub> using the following formula: | |
| − | + | #*:<math>\mbox{T}_{\rm f} = \mbox{T}_{\rm s} + \mbox{D}_{\rm f} \left(\mbox{BHT} - \frac{\mbox{T}_{\rm s}}{\mbox{TD}}\right)</math> | |
| − | + | #*::where: | |
| − | + | #*::: T<sub>s</sub> = average surface temperature (°F) | |
| − | + | #*::: D<sub>f</sub> = depth to the formation (ft) | |
| − | + | #*::: BHT = bottom-hole temperature (found on log header) (°F) | |
| − | + | #*::: TD = total depth (BHT and TD must be from the same log run) (ft) | |
| − | + | # Estimate formation pressure (psi) by multiplying 0.433 (freshwater gradient) by formation depth. | |
| − | :<math>\mbox{T}_{\rm f} = \mbox{T}_{\rm s} + \mbox{D}_{\rm f} \left(\mbox{BHT} - \frac{\mbox{T}_{\rm s}}{\mbox{TD}}\right)</math> | + | # Obtain formation water resistivity R<sub>w</sub> [ohm.m] in one of three ways: |
| − | + | #* From a sample of water from the formation of interest measured for R<sub>w</sub> | |
| − | where: | + | #* Using a water catalog |
| − | * T<sub>s</sub> = average surface temperature (°F) | + | #* Calculating it from an [[SP log]] |
| − | * D<sub>f</sub> = depth to the formation (ft) | ||
| − | * BHT = bottom-hole temperature (found on log header) (°F) | ||
| − | * TD = total depth (BHT and TD must be from the same log run) (ft) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * From a sample of water from the formation of interest measured for R<sub>w</sub> | ||
| − | * Using a water catalog | ||
| − | * Calculating it from an [[SP log]] | ||
| − | |||
==Determine NaCl concentration from r<sub>w</sub>== | ==Determine NaCl concentration from r<sub>w</sub>== | ||
| − | |||
| − | |||
The predominant solute in most formation water is sodium chloride (NaCl). Its concentration determines formation water density and R<sub>w</sub>. When only R<sub>w</sub> is available, we can use NaCl concentration to determine density. | The predominant solute in most formation water is sodium chloride (NaCl). Its concentration determines formation water density and R<sub>w</sub>. When only R<sub>w</sub> is available, we can use NaCl concentration to determine density. | ||
| Line 86: | Line 64: | ||
==Estimate density== | ==Estimate density== | ||
| − | |||
| − | + | Estimate formation water density from ppm NaCl and temperature using the chart in [[:file:formation-fluid-pressure-and-its-application_fig5-6.png|Figure 2]]. The following table describes the procedure to use with the chart. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | + | # Enter the chart along the X-axis using formation temperature. | |
| + | # Proceed vertically to the appropriate salt concentration expected in the zone. | ||
| + | # Proceed horizontally to read the liquid density at atmospheric pressure. | ||
| + | # Using the “Effects of Pressure” segment of the chart, add a density increment to the above-computed density to correct for pressure effects. | ||
==See also== | ==See also== | ||
| Line 121: | Line 85: | ||
[[Category:Critical elements of the petroleum system]] | [[Category:Critical elements of the petroleum system]] | ||
[[Category:Formation fluid pressure and its application]] | [[Category:Formation fluid pressure and its application]] | ||
| + | [[Category:Treatise Handbook 3]] | ||
Latest revision as of 15:19, 13 September 2022
| Exploring for Oil and Gas Traps | |

| |
| Series | Treatise in Petroleum Geology |
|---|---|
| Part | Critical elements of the petroleum system |
| Chapter | Formation fluid pressure and its application |
| Author | Edward A. Beaumont, Forrest Fiedler |
| Link | Web page |
| Store | AAPG Store |
Formation water density is a function of three variables:
- Temperature
- Pressure
- Total dissolved solids (TDS)
It is the mass of the formation water per unit volume of the formation water and is given in metric units (g/cm3). For reservoir engineering calculations, it is considered equivalent to specific gravity.
Estimating density from TDS[edit]
If TDS is known from a chemical analysis of formation water, then the formula below can be used to estimate formation water density (ρw):[1]
Procedure: estimating density from rw[edit]
Use the procedure outlined in the table below to estimate formation water density at reservoir conditions using Rw. The approximate error is ±10% (after [1]).
- Gather data: formation temperature (Tf ), water resistivity (Rw ), and formation pressure. Estimate pressure by multiplying depth by 0.433 psi/ft or other appropriate gradient. Check for Tf errors.
- Estimate sodium chloride (NaCl) concentration from R using Figure 1.
- Estimate density from wt % NaCl and temperature using Figure 2.
Gather data[edit]
To estimate formation water density, collect estimates of the following:
- Formation temperature
- Formation pressure
- Formation water resistivity
- Estimate formation temperature Tf using the following formula:
-
- where:
- Ts = average surface temperature (°F)
- Df = depth to the formation (ft)
- BHT = bottom-hole temperature (found on log header) (°F)
- TD = total depth (BHT and TD must be from the same log run) (ft)
- where:
-
- Estimate formation pressure (psi) by multiplying 0.433 (freshwater gradient) by formation depth.
- Obtain formation water resistivity Rw [ohm.m] in one of three ways:
- From a sample of water from the formation of interest measured for Rw
- Using a water catalog
- Calculating it from an SP log
Determine NaCl concentration from rw[edit]
The predominant solute in most formation water is sodium chloride (NaCl). Its concentration determines formation water density and Rw. When only Rw is available, we can use NaCl concentration to determine density.
Use Figure 1 to determine NaCl concentration. At the intersection of formation temperature (from Y-axis) and Rw (from X-axis), find the NaCl concentration (in ppm) by reading diagonal line labels and interpolating.
Estimate density[edit]
Estimate formation water density from ppm NaCl and temperature using the chart in Figure 2. The following table describes the procedure to use with the chart.
- Enter the chart along the X-axis using formation temperature.
- Proceed vertically to the appropriate salt concentration expected in the zone.
- Proceed horizontally to read the liquid density at atmospheric pressure.
- Using the “Effects of Pressure” segment of the chart, add a density increment to the above-computed density to correct for pressure effects.