Chromatography
| Wiki Write-Off Entry | |
|---|---|

| |
| Author | Reem Alotaibi |
| Affiliation | Saudi Aramco |
| Competition | 2021 Middle East Wiki Write Off |
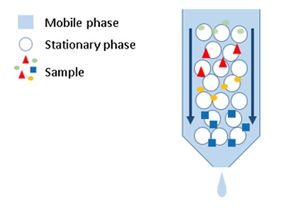
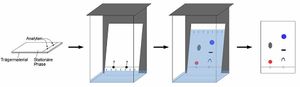
Chromatography is a process for separating components of a mixture. To get the process started, the mixture is dissolved in a substance called the mobile phase, which carries it through a second substance called the stationary phase.
Stationary phase[edit]
The stationary phase in chromatography is composed of either a solid or liquid substance attached to a glass or a metal surface on which the components of the mixture to be separated and there are numerous stationary phases, with different modes of action and requires careful selection by the user based on application.
Mobile phase[edit]
The mobile phase in chromatography is either a liquid or gas, which is passed through a chromatographic column where the components of the mixture are separated at different rates by adsorbing to the stationary phase.
The different components of the mixture travel through the stationary phase at different speeds, causing them to separate from one another. The nature and selection of specific mobile and stationary phases determines which substances travel more quickly or slowly, and how they are separated. The relative “speed” at which components pass through a chromatographic system and is termed retention time.
Types of chromatography:[edit]
Gas chromatography (GC)[edit]
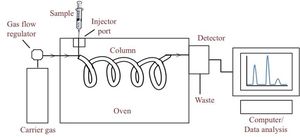
Gas chromatography (GC) is a separation technique in which compounds in a mixture are separated on the basis of chemical differences resulting in increased affinity (or not) of each compound to selected stationary/mobile phases. Typically, the mixture of compounds to be separated is either a liquid or gas that is vaporized in the injection point.
Steps of Gas chromatography[edit]
- The sample is injected into the inlet where it is vaporized into a gaseous state. The vaporized components mix with the mobile phase and are carried through the chromatographic column.
- The column comprises the stationary phase and is where the compound are separated on the basis of their affinity to the stationary phase.
- The components of the mixture reach the detector (there are many GC detectors have been developed e.g. FID, ECD, NPD, SCD etc… which are selected and used based on the application) at different times due to differences in the time they are retained in the column.
Application of Gas chromatography[edit]
- GC is used to calculate the concentration of different chemicals in various samples.
- GC is used in the analysis of air pollutants, oil spills, Food contaminants, drugs of abuse detection etc…
- GC is routinely used in the petroleum industry, spanning applications to the exploration of petroleum resources, refining of finished products to industrial and academic research on new petrochemicals.
Gas chromatography—Mass spectrometry (GC-MS)[edit]
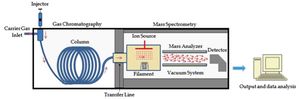
Gas chromatography–mass spectrometry (GC-MS) is hyphenated analytical technique that combines the features of gas chromatography (GC) and mass spectrometry (MS) to detect and identify different substances within samples.
Steps of Gas chromatography—Mass spectrometer:[edit]
- The analysis begins with gas chromatography (GC), once the components leave the GC column (stationary phase), they are ionized and fragmented by the mass spectrometer (MS) using electron (EI) or chemical ionization (CI) sources.
- Ionized molecules and fragments are then accelerated through the instrument’s mass analyzer, which is typically a quadrupole (single Q or triple QQQ) or ion trap.
- Then ions are separated based on their different mass-to-charge (m/z) ratios. GC-MS data acquisition can be performed in either full scan mode, to cover either a wide range of m/z ratios, or selected ion monitoring (SIM) mode, to gather data for specific masses of interest.
- The final steps of the process involve is ion detection and analysis, with fragmented ions appearing as a function of their m/z ratios. Peak areas, meanwhile, are proportional to the quantity of the corresponding compound in the analyzed sample. When a complex sample is separated by GC-MS, it will produce many different peaks in the gas chromatogram and each peak generates a mass spectrum which provides additional information to aid compound identification.
Application of Gas chromatography—Mass spectrometry:[edit]
- GC—MS can be used for a variety of applications such as detection of potential toxic chemicals in foods, quantitation of organic contaminants in water, and analysis of petroleum products during oil processing.
Liquid chromatography[edit]
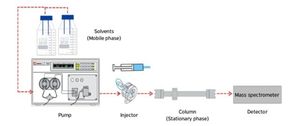
Liquid chromatography (LC) is a separation technique where the mobile phase used is liquid and the sample is introduced as a liquid too, and the separation can take place either in a column or a plain surface (e.g. TLC)
Steps of Liquid chromatography[edit]
- The column or paper is prepared where the stationary phase (cellulose or silica) is applied on the solid support.
- The sample is added to the liquid mobile phase, which is then injected into the chromatographic system.
- The mobile phase moves through the stationary phase before coming out of the column or the edge of the paper.
- An elution solution is applied to the system to separate the molecules from the stationary phase.
Applications of Liquid chromatography[edit]
- Liquid chromatography is an effective method for the separation of a colored solution as they form two separate bands after separation.
- This method can also be used over other techniques as it is quite simple and less expensive.
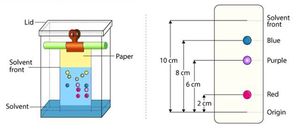
Paper chromatography[edit]
Paper chromatography is a separation technique where the separation is performed on a specialized paper.
Steps of Paper chromatography[edit]
- The stationary phase is selected as a fine quality cellulosic paper.
- Different combinations of organic and inorganic solvents are taken as the mobile phase.
- About 2-200 µl of the sample solution is injected at the baseline of the paper, and it is allowed to air dry.
- The sample loaded paper is then carefully dipped into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the paper, the paper is taken out.
- The retention factor is calculated, and the separated components are detected by different techniques.
Uses of Paper chromatography[edit]
- Paper chromatography is performed to detect the purity of products
- It can also be employed to detect contamination in various samples, like food and beverages.
- This method can also be used for the separation of impurities from various industrial products.
- The analysis of the reaction mixtures in chemical labs is also conducted via paper chromatography.
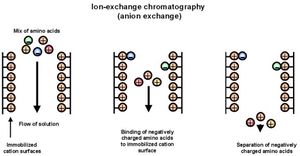
Anion exchange chromatography (IEC)[edit]
Anion exchange chromatography is another chromatographic separation technique which is used for the separation of negatively charged molecules. The mechanism of action is based on compound interaction with a positively charged stationary phase, which is in the form of a ion-exchange resin (IEC).
Steps of Anion exchange chromatography[edit]
- A column packed with positively charged resin is taken as the stationary phase.
- The mixture with the charged particles is then passed down the column where the negatively charged molecules bind to the positively charged resins.
- The anion exchange resin is then passed through the column where the negatively charged molecules now bind to the anion exchange resin displacing the positively charged resin.
- Now an appropriate buffer is applied to the column to separate the complex of anion exchange resins and the charged molecules.
Uses of Anion exchange chromatography[edit]
- Ion exchange chromatography is used in the purification of water where the positively charged ions are replaced by hydrogen ions, and the negatively charged ions are replaced by hydroxyl ions.
- This method also works as an effective method for the analysis of the products formed after hydrolysis of nucleic acids.
- The separation of metals and other inorganic compounds is also facilitated by the ion-exchange chromatography.
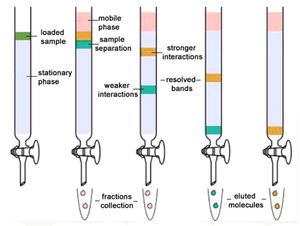
Column chromatography[edit]
Column chromatography is a classical separation technique where components in a mixture are separated on the basis of their differential adsorption with the selected stationary phase, resulting in them moving at different speeds when passed through a column.
It is a solid-liquid chromatography technique in which the stationary phase is a solid and the mobile phase is a liquid or gas.
Steps of Column chromatography[edit]
- The column is prepared by taking a glass tube that is dried and coated with a thin, uniform layer of stationary phase (cellulose, silica).
- Then the sample is prepared by adding the mixture to the mobile phase. The sample is introduced into the column from the top and is allowed to pass the sample under the influence of gravity.
- The molecules bound to the column are separated by elution technique where either solution of the same polarity is used (isocratic technique), or different samples with different polarities are used (gradient technique).
- The separated molecules can further be analyzed for various purposes.
Common applications of Column chromatography[edit]
- Column chromatography is routinely used for the separation of impurities and purification of various biological mixtures.
- This technique can also be used for the isolation of active molecules and metabolites from various samples.
- Column chromatography is increasingly used for the detection of drugs in crude extracts.
- Another application of column chromatography is open column silica-gel used to remove the heavy stuff such as asaphltene from oil sample then separate the maltene into three fractions called saturate, aromatic and risen based on their chemical properties. Each fraction contains different compounds that hold very important information about the oil source and thermal maturity.
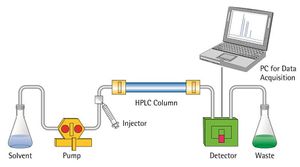
High-performance liquid chromatography[edit]
High-performance liquid chromatography is a modified form of column chromatography where the components of a mixture are separated on the basis of their affinity with the stationary phase.
Steps of HPLC[edit]
- The column is prepared by taking a glass tube that is dried and coated with a thin, uniform layer of stationary phase (cellulose, silica).
- Then the sample is prepared by adding the mixture to the mobile phase. The sample is introduced into the column from the top, and a high-pressure pump is used to pass the sample at a constant rate.
- The mobile phase then moves down to a UV-Vis detector that detects molecules at a certain absorbance wavelength. Numerous other detectors (e.g. DAD, MS, MS/MS, Ion Trap etc…) have been developed and used in conjunction with HPLC systems.
- The separated molecules can further be analyzed for various purposes.
Uses of HPLC[edit]
- High-performance liquid chromatography is used in the analysis of pollutants present in environmental samples.
- It is performed to maintain product purity and quality control of various industrial productions.
- This technique can also be used to separate different biological molecules like proteins and nucleic acids.
- The increased speed of this technique makes the process faster and more effective.
Thin-layer chromatography (TLC)[edit]
Thin-layer chromatography is a separation technique where the stationary phase is applied as a thin layer on a solid support plate with a liquid used as the mobile phase.
Steps of Thin-layer chromatography (TLC)[edit]
- The stationary phase is uniformly applied on the solid support (glass, thin plate or aluminum foil) and dried.
- The sample is injected as spots on the stationary phase about 1 cm above the edge of the plate.
- The sample loaded plate is then carefully dipped into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the plate, the plate is taken out.
- The retention factor is calculated as in paper chromatography, and the separated components are detected by different techniques.
Uses of Thin-layer chromatography (TLC)[edit]
- Thin-layer chromatography is routinely performed in laboratories to identify different substances present in a mixture.
- This technique helps in the analysis of fibers in forensics.
- TLC also allows the assay of various pharmaceutical products.
- It aids in the identification of medicinal plants and their composition.
See also[edit]
- 2021 Middle East Wiki Write Off
- Super basins
- Structural restoration
- Condensate banking effect
- Fault seal analysis for reservoir development
References[edit]
- Ettre L.S Zlatkis A. 75 Years of Chromatography: A Historical Dialouge. Elsevier. 2011.
- Ettre LS, Sakodynskii KI. M. S. Tswett and the discovery of chromatography II: Completion of the development of chromatography (1903-1910). Chromatographia. 1993; 35: 329-338.
- Ebere, E.C., Obinna, I.B., and Wirnkor, V.A.(November 2019). Applications of Column, Paper, Thin Layer and Ion Exchange Chromatography in Purifying Samples.
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University
- Karlsson E, Ryden L, Brewer J Protein purification. Principles, High Resolution Methods, and Applications. Ion exchange chromatography. 2nd ed. New York: Wiley; 1998. [Google Scholar].
- Ó’Fágáin, C., Cummins, P. M., & O’Connor, B. F. (2017). Gel-Filtration Chromatography. Methods in molecular biology (Clifton, N.J.), 1485, 15–25.
- Sparkman DO, Penton Z, Kitson FG (17 May 2011). Gas Chromatography and Mass Spectrometry: A Practical Guide. Academic Press. ISBN 978-0-08-092015-3
- Anupama Sapkota (July 11, 2020). Types of Chromatography (Definition, Principle, Steps, Uses). [ Microbe notes].
- Gas chromatography. PetroWiki. https://petrowiki.spe.org/Gas_chromatography , 5 October 2015 Revision.