Dolomite
| Dolomitization | |

| |
| Series | Course Notes |
|---|---|
| Author | Lyndon S. Land |
| Link | Web page |

Mineralogy
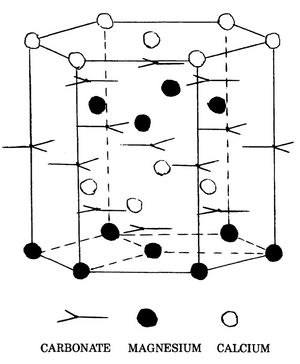
Dolomite is a rhombohedral carbonate with the ideal formula CaMg(CO3)2 in which calcium and magnesium occupy preferred sites. In the ideal mineral, planes of CO3 anions alternate with planes of cations with the c-axis of the crystal perpendicular to the alternating stacked anion and cation planes. Ordering occurs by the additional alternation of cation planes containing only calcium with cation planes containing only magnesium (Figure 1). It is possible to conceive of a mineral having the same composition as ideal dolomite ((Ca0.5Mg0.5)(CO3) in which all cation planes are alike, containing equal numbers of calcium and magnesium ions. Such a mineral is not dolomite. Such a disordered arrangement of ions occupies more volume than that of the ideal dolomite structure and is unstable with respect to an ordered phase.
Perhaps surprisingly, the two compounds just described, ideal dolomite and a disordered 1-to-1 ratio Ca-Mg carbonate, are both rare in sedimentary rocks. Ideal dolomite rarely comprises ancient dolomitic sediments and never modern sediments, and the completely disordered polymorph does not occur at all. The dolomite which does occur in sedimentary rocks is commonly Ca-rich, having compositions which range from about Ca(Ca0.16Mg0.84)(CO3)2 to ideality, and/or exhibits weak, diffuse, X-ray diffraction, suggesting considerably less structural order than its composition should dictate. With respect to ideal dolomite, all such naturally occurring dolomite is metastable, and the capacity exists for reactions to occur toward a more stable (more stoichiometric or better ordered) phase.
The term protodolomite was defined by Graf and Goldsmith[1] as "single-phase rhombohedral carbonates which deviate from the composition of the dolomite that is stable in a given environment, or are imperfectly ordered, or both, but which would transform to dolomite if equilibrium were established." Gaines[2] modified the definition to include only ordered phases. Land[3] recommended that the term be dropped altogether, since almost all sedimentary dolomite is really protodolomite by Gaines'[2] definition. What is important is not what we call these natural materials, but what they really are.
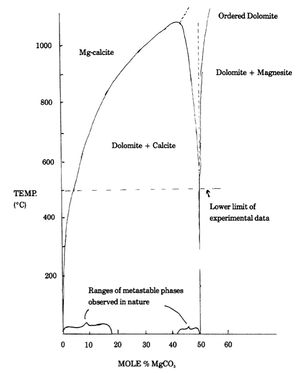
Hydrothermal experiments[1][4] extrapolated to low temperature, demonstrate that calcite and dolomite are essentially ideal in composition at 25°C (Figure 2). In other words, any double carbonate crystal of Ca and Mg at 25°C which is not essentially pure dolomite is either metastable or unstable with respect to a mixture of pure calcite plus pure dolomite. The same thing is true with respect to ideal dolomite plus magnesite. The composition of phases which we observe at Earth's surface define the range of metastability. Unstable phases are only observed as transient states in the laboratory. In the case of dolomite, few phases containing more than about 8% excess calcium (on a molar basis) have been reported to date, although the data are admittedly sparse.
Reeder[5] has shown that the structure of various kinds of dolomite revealed by transmission electron microscopy and electron diffraction can be classified into at least three types. All structures are ordered, although the degree of order is variable and difficult to quantify. The first, characteristic only of Holocene dolomite, consists of irregular "mosaics" on a scale of tens or hundreds of Angstroms. The crystals are characterized by extremely high densities of crystallographic faults and dislocations, and can be thought of as an aggregate of "micro-crystals" whose compositions may vary, forming a very discontinuous lattice. This leads to many unsatisfied or strained chemical bonds and to X-ray diffraction patterns with broad, generally weak reflections. This kind of dolomite is also characterized by large trace element substitutions, especially strontium[6] and sodium.[7] Qualitative data suggest that this material is extremely soluble compared to better ordered forms of dolomite. Land's[8] attempts to beneficiate samples composed of mixtures of this kind of dolomite and aragonite (for example, supratidal crusts from Florida and the Bahamas) by slow leaching in acetic acid resulted in only slight concentration of the dolomite by selective solution of aragonite. CO2 for isotopic analyses of Holocene dolomite is evolved much faster than from finely ground ancient dolomite. All evidence suggests that Holocene dolomite is a unique, highly soluble material. It is clearly a metastable phase, unknown (in an unmodified form) in ancient rocks.
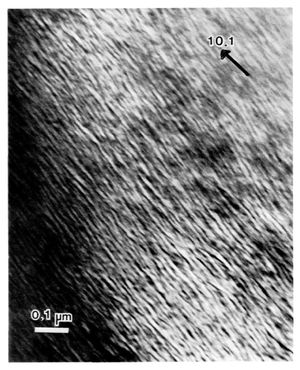
The second and most common kind of sedimentary dolomite exhibits a lamellar or "tweed" structure when examined by transmission electron microscopy and electron diffraction, which Reeder[5] has interpreted as a structural and/or compositional modulation on a scale of several hundred Angstroms (Figure 3). At present this kind of dolomite is thought to consist of two intimately intergrown lamellar domains parallel to the rhomb face with slightly different structures and/or compositions. The texture resembles spinoidal decomposition, or solid state unmixing on a scale of a few hundred angstroms from a single homogeneous precursor. The exact structure and composition of the two domains or lamellae is not known, although one must be more stable (and presumably more magnesium rich) than the other. This type of dolomite is clearly metastable, but continued stabilization cannot proceed spontaneously because it is limited by solid state diffusion. Continued stabilization can occur as a result of solution-reprecipitation processes however, and it has been demonstrated that bulk Ca-rich dolomites dissolve more rapidly than ideal dolomite.[9] Continued stabilization toward a more stoichiometric dolomite would presumably be promoted if pore fluids in the rock changed to enable dissolving out of the less stable, Ca-rich domain. Porosity could easily increase under these conditions.
A third kind of dolomite is nearly ideal in composition, and when examined by transmission electron microscopy and electron diffraction is observed to be homogeneous, consisting of large single domains. This kind of dolomite is presently known mostly from ancient, deeply buried sequences and from metamorphic rocks.
Useful links
References
- ↑ 1.0 1.1 Graf, D. L. and J. R. Goldsmith, 1956, Some hydrothermal syntheses of dolomite and protodolomite: Jour. Geology, v. 64, p. 173-186.
- ↑ 2.0 2.1 Gaines, A. M., 1977, Protodolomite redefined: Jour. Sed. Petrology, v. 47, p. 543-546.
- ↑ Land L. S., 1980, The isotopic and trace element geochemistry of dolomite; the state of the art, in D. H. Zenger, J. B. Dunham, and R. L. Ethington, eds., Concepts and models of dolomitization: SEPM Spec. Pub. No. 28, p. 87-110.
- ↑ Goldsmith, Jr., and H. C. Heard, 1961, Subsolidus phase relations in the system CaCO3-MgCO3: Jour. Geology, v. 69, p. 45-74.
- ↑ 5.0 5.1 Reeder, R. J., 1981, Electron optical investigation of sedimentary dolomites: Contr. Mineralogy Petrology, v. 76. p. 148-157.
- ↑ Behrens E. W., and L. S. Land, 1972, Subtidal Holocene dolomite, Baffin Bay, Texas: Jour. Sed. Petrology, v. 42, p. 155-161.
- ↑ Land L. S., and G. K. Hoops, 1973, Sodium in carbonate sediments and rocks; a possible index to the salinity of diagenetic solutions: Jour. Sed. Petrology, v. 43, p. 614-617.
- ↑ Land, Lyndon S., 1982, Dolomitization: AAPG Course Notes 24, p. 1-20.
- ↑ Busenberg, E. and L. N. Plummer, 1982, The kinetics of dissolution of dolomite in CO2-H2O systems at 1.5 to 65°C and 0 to 1 atm Pco2: Am. Jour. Sci., v. 282, p. 45-78.