Rock-Eval pyrolysis
| Exploring for Oil and Gas Traps | |

| |
| Series | Treatise in Petroleum Geology |
|---|---|
| Part | Critical elements of the petroleum system |
| Chapter | Evaluating source rocks |
| Author | Carol A. Law |
| Link | Web page |
| Store | AAPG Store |
What is pyrolysis?
Pyrolysis is the decomposition of organic matter by heating in the absence of oxygen. Organic geochemists use pyrolysis to measure richness and maturity of potential source rocks. In a pyrolysis analysis, the organic content is pyrolyzed in the absence of oxygen, then combusted. The amount of hydrocarbons and carbon dioxide released is measured. The most widely used pyrolysis technique is Rock-Eval.
Rock-eval pyrolysis
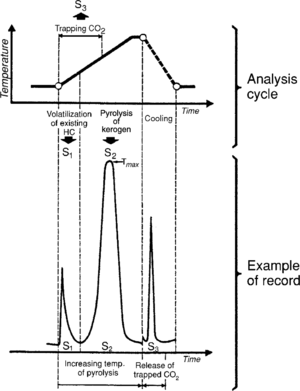
In Rock-Eval pyrolysis, a sample is placed in a vessel and is progressively heated to temperature::550°C under an inert atmosphere. During the analysis, the hydrocarbons already present in the sample are volatized at a moderate temperature. The amount of hydrocarbons are measured and recorded as a peak known as S1. Next pyrolyzed is the kerogen present in the sample, which generates hydrocarbons and hydrocarbon-like compounds (recorded as the S2 peak), CO2, and water.[1] The CO2 generated is recorded as the S3 peak. Residual carbon is also measured and is recorded as S4.
Figure 1 shows the cycle of analysis and the corresponding recording.
Pyrolysis indices
The table below lists the Rock-Eval pyrolysis peaks and explains what they represent.
| Peak | Is a measurement of… | Comment |
|---|---|---|
| S1 mg Hc/g rock | The free hydrocarbons present in the sample before the analysis | Can be thought of as a residual hydrocarbon phase. When S1 is large relative to S2, an alternative source such as migrated hydrocarbons or contamination should be suspected |
| S2 mg Hc/g rock | The volume of hydrocarbons that formed during thermal pyrolysis of the sample | Used to estimate the remaining hydrocarbon generating potential of the sample |
| S3 mg Co2/g rock | The CO2 yield during thermal breakdown of kerogen | Most prevalent in calcareous source rocks. |
| S4 mg carbon/g rock | The residual carbon content of the sample | Residual carbon content of sample has little or no potential to generate hydrocarbons due to a lack of hydrogen and the chemical structure of the molecule |
Estimating total organic carbon with pyrolysis
The percent total organic carbon (TOC) is actually a value that is calculated, not measured directly, using the following formula:
Units are usually given as wt % organic carbon per weight of dry rock (milligrams hydrocarbon per gram of rock).
See also
- Source rock richness
- LECO method in estimating richness
- Using conventional well logs to estimate richness
![{\displaystyle \%{\mbox{TOC}}=[0.082({\mbox{S1}}+{\mbox{S2}})+{\mbox{S4}}]/10}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a345364ae63ec45e62448597ecce64b02ce1be30)