Carbon dioxide (CO2) sequestration
| Carbon dioxide sequestration in geological media: State of the science | |

| |
| Series | Studies in Geology |
|---|---|
| Chapter | Geological input to selection and evaluation of CO2 geosequestration sites |
| Author | John G. Jkaldi, Catherine M. Gibson-Poole, Tobias H. D. Payenberg |
| Link | Web page |
| PDF file (requires access) | |
| Store | AAPG Store |
Coal, oil, and natural gas currently supply about 85% of the world's energy needs. Moreover, given the relatively low cost and abundance of fossil fuels together with the huge sunken investment in fossil-fuel-based infrastructure, fossil fuels will likely continue to be used for at least the next 25 to 50 years. The burning of fossil fuels is, however, the major source of anthropogenic (man-made) carbon dioxide (CO2). Carbon dioxide is the main greenhouse gas released to the atmosphere.[1]
Geosequestration, also known as carbon capture and storage (CCS), is a means to reduce anthropogenic CO2 emissions to the atmosphere. Geosequestration involves the long-term storage of captured CO2 emissions in deep subsurface geological reservoirs. Carbon sequestration can be pursued as part of a portfolio of greenhouse gas abatement options, when this portfolio also includes improving the conservation and efficiency of energy use and utilizing nonfossil energy forms such as renewable (solar, wind, and tidal) and nuclear energy.[2] Geosequestration may contribute significant reductions to anthropogenic CO2 emissions. Estimates by the Intergovernmental Panel on Climate Change indicate that a technical potential of at least about 2000 billion metric tonnes of CO2 storage capacity in geological formations likely exists (Table 1).[1]
| Reservoir type | Lower estimate of storage capacity (Gt CO2 | Upper estimate of storage capacity (Gt CO2 |
|---|---|---|
| Oil and gas fields | 675** | 900** |
| Unmineable coal seams in ECBM*** recovery | 3-15 | 200 |
| Deep saline formations | 1000 | Uncertain but possibly 10,000 |
* The storage capacity includes storage options that are not economical.[1]
**These numbers would increase by 25% if undiscovered oil and gas fields were included in this assessment.
***ECBM = enhanced coalbed methane.
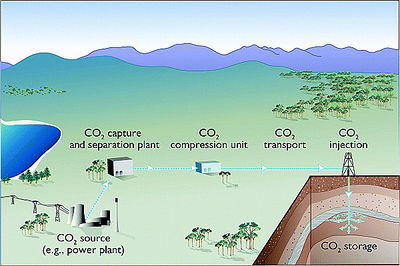
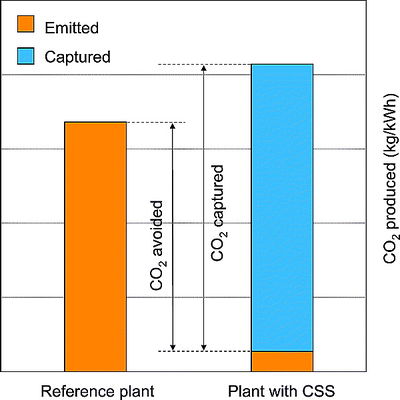
Geosequestration comprises several steps: first, the CO2 is captured at the source, which can be a power plant or other industrial facility; the captured CO2 is then transported, typically via pipeline, from the source to the geological storage site; next, the CO2 is injected deep underground via wells into the geological reservoir; and finally, the CO2 is stored in the geological reservoir, where its movement is carefully monitored and the quantity stored is regularly verified (Figure 1). The capture, transport, and injection processes do require additional energy to be expended (and hence more CO2 is emitted); however, the net CO2 emission reduction is still a significantly large volume to make deep reductions in anthropogenic greenhouse gas emissions. For example, a power plant with CCS could reduce net CO2 emissions to the atmosphere by approximately 80–90% compared to a plant without CCS (Figure 2).[1]
Carbon dioxide capture
Carbon dioxide capture can be conducted at a point (stationary) source of CO2 such as a power plant. Carbon dioxide capture involves capturing the produced CO2 instead of allowing it to be released to the atmosphere. This captured CO2 is then compressed to make it more dense and much easier, and less costly, to transport to the geological storage site.
Anthropogenic CO2 that can be captured is produced by three main types of activity: industrial processes, electricity generation, and hydrogen (H2) production. Industrial processes that lend themselves to CO2 capture include natural gas processing, ammonia production, and cement manufacturing. Note, however, that the total quantity of CO2 produced by these processes is limited. A far larger source, accounting for one-third of total CO2 emissions, is fossil-fueled power production. The types of power plants that are best suited to CO2 capture are pulverized coal (PC), natural gas combined cycle (NGCC), and integrated gasification combined cycle (IGCC) plants.[4] Finally, a potentially large future source of CO2 for capture will be [[H2 production]], whereby the produced H2 is used to fuel a hydrogen economy, i.e., it is used in turbines to produce electricity and in fuel cells to power cars. Technologies for capturing CO2 from electricity generation fall into two general categories: postcombustion and precombustion.[5]
Carbon dioxide transport
Carbon dioxide transport involves moving, or transporting, the captured CO2 from the CO2 point source to the geological storage site. The CO2 is typically transported in a compressed form via pipeline, although the CO2 could also be transported by truck, rail, or in the case of a geological storage site located offshore, ocean tanker.
Carbon dioxide injection
Carbon dioxide injection involves taking the CO2 from the surface and injecting it deep underground into a reservoir rock. The CO2 is injected into the reservoir via a single well or array of wells. Both enhanced oil recovery (EOR) using carbon dioxide floods and acid gas injection (AGI) are mature technologies that involve significant quantities of CO2 being injected underground and are therefore very good analogs for CO2 injection as part of geosequestration activities. The first project using CO2 for EOR began in 1972, and by 1999, 84 operational projects worldwide existed (72 in the United States) injecting an estimated total of more than 15 million tonnes of CO2 per year.[6]
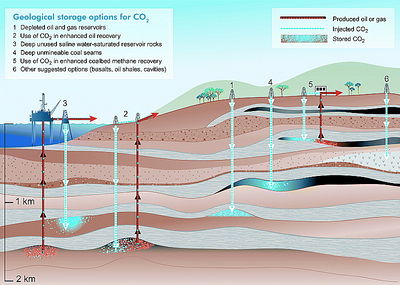
Carbon dioxide storage
Carbon dioxide storage involves keeping the CO2 secured deep underground in a geological reservoir. Carbon dioxide can be stored geologically in a variety of different options (Figure 3). These include depleted oil and gas fields, EOR, deep saline formations, deep unmineable coal seams, enhanced coalbed methane recovery (ECBMR), and other opportunities such as salt caverns.[7][8][9][1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Intergovernmental Panel on Climate Change (IPCC), 2005, IPCC special report on carbon dioxide capture and storage, prepared by Working Group III of the IPCC (B. Metz, O. Davidson, H. C. de Connick, M. Loos, and L. A. Meyer, eds): New York, Cambridge University Press, 442 p.
- ↑ Kaldi, J. G., 2005, Geosequestration: Australian Institute of Geoscientists Quarterly Newsletter, v. 80, p. 1–6.
- ↑ 3.0 3.1 3.2 Kaldi, J. G., C. M. Gibson-Poole, and T. H. D. Payenberg, 2009, Geological input to selection and evaluation of CO2 geosequestration sites, in M. Grobe, J. C. Pashin, and R. L. Dodge, eds., Carbon dioxide sequestration in geological media—State of the science: AAPG Studies in Geology 59, p. 5–16.
- ↑ Davison, J., R. M. Domenichin, and L. Mancuso, 2006, CO2 capture in low rank coal power plants, in N. A. Rokke, O. Bolland, and J. Gale, eds., Greenhouse gas control technologies: Proceedings of the 8th International Conference on Greenhouse Gas Control Technologies: Trondheim, Norwegian University of Science and Technology (NTNU), SINTEF Technology (SINTEF), and International Energy Agency (IEA) Greenhouse Gas RampD Program, 6 p.
- ↑ Kentish, S., B. Hooper, G. Stevens, J. Perera, and G. Qiao, 2006, An overview of technologies for carbon capture: Proceedings of the Australian Institute of Energy National Conference, November 27–29, 2006, Melbourne, 8 p.
- ↑ Electric Power Research Institute (EPRI), 1999, Enhanced oil recovery scoping study, final report, October 1999, TR-113836 (accessed August 10, 2007).
- ↑ Cook, P. J., 1998, Carbon dioxide—Putting it back where it came from: Australian Gas Journal, p. 40–41.
- ↑ Bachu, S., and W. D. Gunter, 1999, Storage capacity of CO2 in geological media in sedimentary basins with application to the Alberta Basin, in P. Reimer, B. Eliasson, and A. Wokaun, eds., Greenhouse gas control technologies: Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, August 30–September 2, 1998, Interlaken, Switzerland, Elsevier, p. 195–200.
- ↑ Cook, P. J., A. J. Rigg, and J. Bradshaw, 2000, Putting it back from where it came from: Is geological disposal of carbon dioxide an option for Australia: The Australian Petroleum Production and Exploration Association (APPEA) Journal, v. 40, no. 1, p. 654–666.