Difference between revisions of "Biodegradation of crude oil in reservoirs"
Cwhitehurst (talk | contribs) |
Cwhitehurst (talk | contribs) |
||
| Line 7: | Line 7: | ||
[[file:Wiki-goldmedal.jpeg|frameless|right]] | [[file:Wiki-goldmedal.jpeg|frameless|right]] | ||
| − | Crude oil is a complex mixture containing tens of thousands of compounds mixed together and the major part are hydrocarbons. Hydrocarbons (HCs) are defined as compounds that only contain carbon and hydrogen without any functional groups. This gives them stability and low reactivity. Under the right conditions, living organisms such as bacteria, algae or fungi are able to consume hydrocarbons as an energy source. The process is called hydrocarbon biodegradation. Hydrocarbon biodegradation by living organisms is a well-known alteration process and the majority of crude oils in the world are biodegraded to different degrees<ref name="Roadifer">Roadifer, R. E., 1987, [https://archives.datapages.com/data/specpubs/methodo2/data/a081/a081/0001/0000/0003.htm Size distributions of the world’s largest known oil and tar accumulations], ''in'' R. F. Meyer, ed., [https://archives.datapages.com/data/alt-browse/aapg-special-volumes/sg25.htm Exploration for heavy crude oil and natural bitumen]: AAPG Studies in Geology 25, p. | + | Crude oil is a complex mixture containing tens of thousands of compounds mixed together and the major part are hydrocarbons. Hydrocarbons (HCs) are defined as compounds that only contain carbon and hydrogen without any functional groups. This gives them stability and low reactivity. Under the right conditions, living organisms such as bacteria, algae or fungi are able to consume hydrocarbons as an energy source. The process is called hydrocarbon biodegradation. Hydrocarbon biodegradation by living organisms is a well-known alteration process and the majority of crude oils in the world are biodegraded to different degrees<ref name="Roadifer">Roadifer, R. E., 1987, [https://archives.datapages.com/data/specpubs/methodo2/data/a081/a081/0001/0000/0003.htm Size distributions of the world’s largest known oil and tar accumulations], ''in'' R. F. Meyer, ed., [https://archives.datapages.com/data/alt-browse/aapg-special-volumes/sg25.htm Exploration for heavy crude oil and natural bitumen]: AAPG Studies in Geology 25, p. 3–23.</ref>. The degree of hydrocarbon biodegradation is dependent on many factors including salinity, hydrocarbon composition, temperature, pH, pressure and moisture. |
==Biodegradation of hydrocarbons definition== | ==Biodegradation of hydrocarbons definition== | ||
| − | Biodegradation of hydrocarbons is an alteration process by living organisms either aerobically and anaerobically (i.e. with or without the presence of oxygen) affecting organic matter. This process can occur in petroleum reservoirs or during migration at shallow depths where water contact is possible and subsurface temperatures are below 80 oC because this temperature is considered as the cutoff temperature where micro-organisms are absent beyond it. [ | + | Biodegradation of hydrocarbons is an alteration process by living organisms either aerobically and anaerobically (i.e. with or without the presence of oxygen) affecting organic matter. This process can occur in petroleum reservoirs or during migration at shallow depths where water contact is possible and subsurface temperatures are below 80 oC because this temperature is considered as the cutoff temperature where micro-organisms are absent beyond it.<ref name="Petersetal">Peters, K. E., D. J. Curry, and M. Kacewicz, 2012, [https://archives.datapages.com/data/specpubs/hedberg4/INTRODUCTION/INTRODUCTION.HTM Introduction: An overview of basin and petroleum system modeling: Definitions and concepts], ''in'' K. E. Peters, D. J. Curry, and M. Kacewicz, eds., [https://archives.datapages.com/data/alt-browse/aapg-special-volumes/hed4.htm Basin modeling: New horizons in research and applications]: AAPG Hedberg Series 4, p. 1–16.</ref> |
==Crude oil Composition== | ==Crude oil Composition== | ||
| Line 71: | Line 71: | ||
| − | [2] | + | [2] |
[3] https://www.e-education.psu.edu/fsc432/content/paraffins | [3] https://www.e-education.psu.edu/fsc432/content/paraffins | ||
Revision as of 15:12, 25 April 2022
| Wiki Write-Off Entry | |
|---|---|

| |
| Author | Mohammed Y. Aljezan |
| Affiliation | Saudi Aramco |
| Competition | 2021 Middle East Wiki Write Off |
Crude oil is a complex mixture containing tens of thousands of compounds mixed together and the major part are hydrocarbons. Hydrocarbons (HCs) are defined as compounds that only contain carbon and hydrogen without any functional groups. This gives them stability and low reactivity. Under the right conditions, living organisms such as bacteria, algae or fungi are able to consume hydrocarbons as an energy source. The process is called hydrocarbon biodegradation. Hydrocarbon biodegradation by living organisms is a well-known alteration process and the majority of crude oils in the world are biodegraded to different degrees[1]. The degree of hydrocarbon biodegradation is dependent on many factors including salinity, hydrocarbon composition, temperature, pH, pressure and moisture.
Biodegradation of hydrocarbons definition[edit]
Biodegradation of hydrocarbons is an alteration process by living organisms either aerobically and anaerobically (i.e. with or without the presence of oxygen) affecting organic matter. This process can occur in petroleum reservoirs or during migration at shallow depths where water contact is possible and subsurface temperatures are below 80 oC because this temperature is considered as the cutoff temperature where micro-organisms are absent beyond it.[2]
Crude oil Composition[edit]
Crude oil consists of four classes (SARA): Saturates: Linear or branched chains of hydrocarbons with only single bonds connecting the atoms (also called alkanes). (e.g. Propane and Iso-butane). [3] Aromatics: Chemical geometry contains of at least one unsaturated hydrocarbon cyclic with single and double bonds in alternating order (e.g. Benzene and Toluene). [4] Resins: Are similar in terms of composition to asphaltenes but different in terms of percentage of presence of atoms relative to each other (i.e. H/C ratio is higher in resins than in asphaltenes). [5] Asphaltenes: Is more complex organic compounds that are considered as the heaviest and most polar constituent in crude oil. Asphaltenes consist from Carbon, Hydrogen, Sulfur, Nitrogen, Oxygen, Vanadium and Nickle (e.g. phenols, fatty acids, esters and porphyrins). [5] Alkanes are generally biodegraded faster than aromatics. This is because aromatics requires more energy to be consumed than alkanes. Furthermore, high molecular weight molecules are more difficult to be biodegraded than low molecular weight molecules.
How does biodegradation occur?[edit]
Bacteria, fungi and algae are the major living organisms that consumes hydrocarbon in different efficiencies with bacteria to be the most efficient contributor. Bacteria are selective towards consuming hydrocarbons. For example, Burkholderia is one type of bacteria which causes alkylaromatic degradation, on the other hand, Acinetobacter sp. leads to C10-C40 linear alkane degradation. [6]
Factors affecting biodegradation of hydrocarbons[edit]
Temperature is an important factor affecting hydrocarbon biodegradation rate. The optimum temperatures for hydrocarbon biodegradation are dependent on the environment of the hydrocarbons. For instance, Figure 1 shows that the highest biodegradation rate for soil environment will occur between 30-40 oC, for freshwater environment between 20-30 oC and for marine environment between 15-20 oC.[6]
Besides temperature, nutrient supply is a very crucial element controlling hydrocarbon biodegradation process. The concentrations of these nutrients will vary depending on the environment. For example, nitrogen, phosphorus and potassium present in low levels in freshwater wetlands due to the high demand of these elements by the plants. On the other hand, the presence of surplus nutrients can negatively impact the hydrocarbon biodegradation process.
What is the mechanism of hydrocarbon biodegradation?[edit]
There are two main pathways for hydrocarbon biodegradation. The first occurs in the presence of oxygen (aerobic). The second process can occur in the absence of oxygen (anaerobic) The aerobic mechanism is highlighted in figure 2.
The normal alkane (C1-C8 n-alkane) in figure 2 will react with oxygen with the help of monooxygenase enzyme produced by living organisms (e.g. bacteria), and converts the normal alkane to an alcohol and oxidize iron from Fe2+ to Fe3+. Under aerobic conditions, simple aromatics such as benzene, xylene and toluene can be degraded. Typically, this requires 3mg/L of dissolved oxygen to degrade 1 mg/L of these aromatics (i.e. 3:1 ratio). If the dissolved oxygen content is lower than 3:1 then the biodegradation rate is slower.
Compared to aerobic degradation, anaerobic degradation is considered to proceed much slower This is because anaerobic pathways require more energy, i.e. they are energetically unfavorable. There are three different pathways that anaerobic microbes can utilize to biodegrade hydrocarbons. All three pathways require inserting an oxidizing group into the molecule, which makes it more active and thus easier to transform to microbial-consumable products such as fatty acids. The first pathway is called fumarate addition and this pathway is used by bacteria to activate C3 to C20 alkanes and alkyl-substituted aromatics such as xylene and toluene. The mechanism proceeds via addition to the double bond by terminal or pre-terminal alkyl group from the alkanes or alkyl-substituted aromatics then followed by the removal of a carbon dioxide molecule (fig.3) [7][8].
Oxygen-independent hydroxylation is another pathway that selected microbes can utilize to activate hydrocarbons in the absence of oxygen. This is illustrated below using ethylbenzene as an example (Figure 4). The process will start with the attack of a hydroxyl group (-OH) to the closest carbon atom to the ring with the help of ethylbenzene dehydrogenase enzyme, forming (S)-1-phenylethanol. Then a molecule of carbon dioxide will react with (S)-1-phenylethanol with the help of (S)-1-phenylethanol dehydrogenase enzyme to form benzoylacetate. The last two steps of the process involve reaction with a thiol group that contains co-enzyme A (CoA). The first thiol attack will convert benzoylacetate to benzoylacetyl-CoA. The second thiol attack will cleave the benzoylacetyl-CoA to benzoyl-CoA and acetyl-CoA (fig.4) [7][9][10][11].
The third pathway is carboxylation. Carboxylation of non-branched alkyl chains the need of the presence of an alkyl group attached to it (e.g. benzene and naphthalene). The exact carboxylation reaction mechanism is still debated, but it is agreed that carbon dioxide is attached directly to the aromatic or aliphatic hydrocarbon via a carboxylase enzyme, requiring the presence of iron and nitrate reducing conditions [12].
How biodegradation of hydrocarbons is identified?[edit]
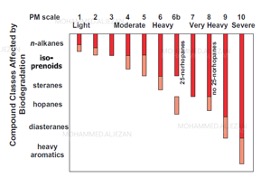
Crude oils that has been biodegraded typically has a lower API gravity, high sulfur content (%S) and vanadium & nickel contents than corresponding non-biodegraded crude oils [13]. Gas Chromatography (GC) analysis of crude oils is commonly used to identify and assess hydrocarbon biodegradation. GC utilizes chromatography to firstly separate individual hydrocarbon components within crude oil mixtures and a detector (either flame ionization or mass spectrometer) to measure the amount of the separated hydrocarbons which are as relative intensities thus allowing comparison between different peak intensities (e.g. low peak intensity will indicate low amounts and high peak intensity will indicate high amounts). In general, microbes consume the simplest hydrocarbons first, i.e. normal alkanes starting from C4 then C5 then C6 and then of increasing chain length. At more advanced levels of biodegradation, branched hydrocarbons (i.e. alkyled) are biodegraded. The general sequence of hydrocarbon biodegradation, by compound class is shown this Figure 5 below.
Biodegradation scales[edit]
In order to assess the level of crude oil biodegradation, a scale has been developed by Peters and Moldowan (PM) [13]. The scale is called the PM scale. The PM scale ranges from 1 to 10, with 10 to be the most altered i.e. most biodegraded. This scale is efficient and can be evaluated in conjunction with observation of the corresponding gas chromatography traces of crude oil samples. This scale illustrates very clearly that microbes favor consumption of simple hydrocarbons starting from n-alkanes and proceed to heavy aromatics at higher levels of biodegradation (fig.5).
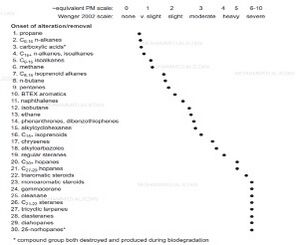
Other authors have proposed their own biodegradation scales, e.g. Another biodegradation scale is Wenger et al. although that scale only describes the oil based on very slight, slight, moderate, heavy and severe biodegradation levels. The scale is based on the presence or absence of specific compound classes (fig.6). The main problem with these scales is that, there are insufficient changes observable in chemical classes at very high biodegradation levels (heavy to severe) thus biodegradation between PM 5-8 on the PM scale and heavy to severe on Wenger et al. scale.
What are the effects of biodegradation on crude oil economically?[edit]
The quality of oil can typically be evaluated based on API gravity, sulfur & metal contents, viscosity and asphaltenes content. Biodegradation is well known to lower the quality of crude oil resulting in lower API gravity, high sulfur and metal contents, which will negatively affect the price of oil since such oils have higher production and refining costs. Biodegradation negatively affects oil production since additional additives are needed to increase the volume of recoverable oil due to its low API gravity and higher viscosity. Furthermore, downstream costs are also higher since they require the removal of the high sulfur and metal contents, which poison catalysts and can corrode pipelines.
Biodegradation of soil or marine hydrocarbon-contaminated environments:[edit]
Despite the fact that biodegraded crude oils can be a disadvantage for crude oil economics, hydrocarbon biodegradation can be implemented as a useful process to deal with hydrocarbon spills in marine and/or soil environments. Many studies have monitored oil biodegradation in the natural environment [14], and oil spill remediation strategies include procedures to actively stimulate microbes to biodegrade spilt oil in the environment. Most reported remediation strategies attempt to introduce known hydrocarbon-consuming bacteria to these environments. Numerous studies report the use of surfactants along with these bacteria in order to break large oil droplets to smaller micelles to increase the contact area, thus, increase the rate of biodegradation [14].
References[edit]
[1]
[2]
[3] https://www.e-education.psu.edu/fsc432/content/paraffins
[4] https://www.e-education.psu.edu/fsc432/content/aromatic-hydrocarbons
[5] https://ogst.ifpenergiesnouvelles.fr/articles/ogst/pdf/2004/05/speight1_vol59n5.pdf
[6] https://www.hindawi.com/journals/btri/2011/941810/
[7] Fuchs, G., Boll, M. and Heider, J., 2011. Microbial degradation of aromatic compounds-from one strategy to four.
[8] Bian, X.Y., Mbadinga, S.M., Liu, Y.F., Yang, S.Z., Liu, J.F., Ye, R.Q., Gu, J.D. and Mu, B.Z., 2015. Insights into the anaerobic biodegradation pathway of n-alkanes in oil reservoirs by detection of signature metabolites. [9] Heider, J., 2007. Adding handles to unhandy substrates: anaerobic hydrocarbon activation mechanisms. [10] Boll, M., Loffler, C., Morris, B.E. and Kung, J.W., 2014. Anaerobic degradation of homocyclic aromatic compounds via arylcarboxyl-coenzyme A esters: organisms, strategies and key enzymes.
[11] Callaghan, A.V., 2013. Enzymes involved in the anaerobic oxidation of n-alkanes: from methane to long-chain paraffins.
[12] Biocatalytic carboxylation - Chemical Society Reviews (RSC Publishing)
[13] Moldowan, M. J., Peters, K. E., & Walters, C. C. (2007). Biodegradation parameters. In Biomarker guide: Volume 2, biomarkers and isotopes in petroleum systems and earth history (Second, Vol. 2, pp. 645–705). essay, Cambridge University Press.
[14] Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review (nih.gov)
[15] A practical biodegradation scale for use in reservoir geochemical studies of biodegraded oils - ScienceDirect
- ↑ Roadifer, R. E., 1987, Size distributions of the world’s largest known oil and tar accumulations, in R. F. Meyer, ed., Exploration for heavy crude oil and natural bitumen: AAPG Studies in Geology 25, p. 3–23.
- ↑ Peters, K. E., D. J. Curry, and M. Kacewicz, 2012, Introduction: An overview of basin and petroleum system modeling: Definitions and concepts, in K. E. Peters, D. J. Curry, and M. Kacewicz, eds., Basin modeling: New horizons in research and applications: AAPG Hedberg Series 4, p. 1–16.