Early carbonate diagenesis
| Exploring for Oil and Gas Traps | |

| |
| Series | Treatise in Petroleum Geology |
|---|---|
| Part | Predicting the occurrence of oil and gas traps |
| Chapter | Predicting reservoir system quality and performance |
| Author | Dan J. Hartmann, Edward A. Beaumont |
| Link | Web page |
| Store | AAPG Store |
Diagenetic environments
Early diagenesis occurs at the surface or in the shallow subsurface. It is usually responsible for much of the cementation of carbonate sediments. Early diagenesis occurs in a consistent sequence of four diagenetic zones:
- Marine phreatic
- Vadose
- Freshwater phreatic
- Mixing
Each zone has characteristic cement textures and alteration features. The sequence of diagenesis usually follows the above order; however, a rock may be exposed to a variety of environments many different times, making diagenetic sequence interpretation difficult.
Summary diagram
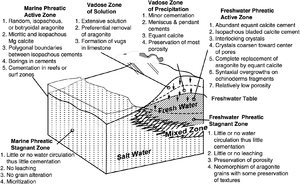
The block diagram in Figure 1 summarizes early diagenetic processes and products that occur in carbonate environments.
Marine phreatic environment
The marine phreatic environment, in most cases, is where carbonate sediments originate and begin their diagenetic history. It can be divided into two distinct zones that represent two ends of a spectrum:[1]
- Active marine zone where water flow combined with other factors result in precipitation of aragonite or magnesium (Mg) calcite.
- Stagnant marine zone in which there is little or no water movement and consequently no cementation or sediment alteration.
Active marine phreatic zone
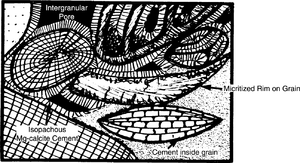
The cementation rate is greatest in the active marine phreatic environment where three conditions occur:
- pH increases above 9 due to photosynthesis and respiration of a reef biomass
- CO2 degassing
- Waves, tides, or currents force water through pores (works best at shelf margins where buildup is present or along shoreface)
Magnesium calcite or aragonite are the only cements that precipitate in the active marine phreatic zone. Both are unstable in Mg-deficient water regardless of whether it is marine, brackish, or fresh and tend to alter to low-Mg calcite because most water is magnesium deficient. Their common form is isopachous coatings on grains. Micritization of grains occurs in the active marine phreatic zone.
The diagram in Figure 2 summarizes the diagenesis of this zone.
Stagnant marine phreatic
Micritization and minor intragranular cementation are the only diagenetic processes occurring in the stagnant marine phreatic zone. Cementation is limited because of little or no water movement. Micritization by boring algae occurs at the sediment–water interface to length::1 m below the interface.[1] Sediments deposited in lagoons, below the wave base on carbonate ramps, or on a debris slope have diagenetic histories that begin in the stagnant marine phreatic zone.
Vadose zone
The vadose zone extends from the land surface to the water table. The pore space of sediments in the vadose zone contains both air and water. Solution of carbonate sediment or rock occurs in the vadose zone as a result of meteoric water movement. Initially, meteoric water is undersaturated with CaCO3 but it quickly becomes saturated as it moves downward and dissolves carbonate grains or cements. Organic matter in the soil zone produces CO2. It combines with the water to aid in the solution process. Saturated meteoric water precipitates calcite through evaporation or CO2 degassing.
Climate greatly affects vadose diagenesis. In arid climates diagenetic alteration may be limited, whereas in humid climates it may be extensive. In humid climates, a thick soil zone develops and there is abundant meteoric water.
The vadose zone can be divided into three zones:[1]
- Soil zone
- Solution zone
- Precipitation zone
Vadose solution zone
The vadose solution zone extends tens to hundreds of meters below the surface, depending on associated relief of nearby land. Any form of calcium carbonate may dissolve in the vadose solution zone. Aragonite grains are especially susceptible to dissolution. Caves may form with prolonged exposure. Distinguishing leaching in the vadose solution zone from solution in the freshwater phreatic zone is difficult.[1]
Vadose precipitation zone
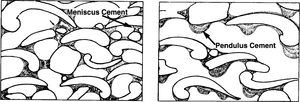
The vadose precipitation zone begins where water in the vadose zone becomes saturated with CaCO3. Slight temperature increases or CO2 degassing causes calcite to precipitate. Cementation is generally minor and reflects pore-water distribution. Meniscus cement precipitates where water clings between grains in a meniscus manner. Pendulous or microstalactitic cement precipitates where water droplets form underneath grains. Cement tends to be very fine equant calcite crystals. If magnesium is present in pore water, then calcite precipitation may be inhibited and aragonite or even dolomite may precipitate.[1]
The sketches in Figure 3 illustrate recent limestones from the intertidal-supratidal zone. They show petrographic aspects of vadose precipitation zone cements.
Freshwater phreatic zone
The freshwater phreatic zone (|Figure 4) lies between the vadose and mixing zones. It is 100% saturated with fresh water. Water enters the zone through the vadose zone or directly enters the zone through streams and lakes. Rainfall amounts, pore type, and relief determine its size and geometry. It is generally lens shaped. Climatic and sea level changes cause the zone to be dynamic. It changes shape with changes in rainfall amounts or sea level. In arid climates the zone can be completely missing. As a rule of thumb, for every meter the zone lies above sea level, there are length::32 m of fresh water below sea level.
Active and stagnant zones
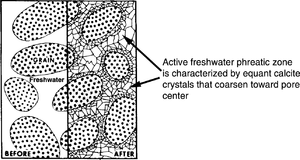
Based on diagenesis, the freshwater phreatic zone can be divided into two major zones:
- Active freshwater phreatic zone
- Stagnant freshwater phreatic zone
The active freshwater phreatic zone is where freshwater movement occurs in the phreatic zone. Meteoric water that enters the phreatic zone without passing through the vadose is undersaturated with respect to CaCO3 but becomes saturated as it dissolves grains. Based on CaCO3 saturation, the active freshwater phreatic zone may be subdivided into undersaturated and saturated zones. In the undersaturated zone, solution occurs, creating moldic or vuggy porosity. In the active saturated freshwater phreatic zone extensive and rapid cementation occurs. Cement is equant calcite that coarsens toward pore centers. Syntaxial overgrowths on echinoderm fragments are common.
The stagnant freshwater phreatic zone occurs where there little to no movement. Pore water is near equilibrium with surrounding rock and lack of water movement means little cementation occurs. Consequently, primary porosity is generally preserved.
See also
- Predicting carbonate porosity and permeability
- Carbonate facies
- Carbonate diagenesis
- Carbonate diagenetic stages
- Basics of carbonate porosity formation and preservation
- Sea level cycles and carbonate sequences
- Sea level cycles and carbonate diagenesis
- Sea level cycles and climate
- Sequences during low-amplitude, high-frequency cycles
- Sequences during moderate-amplitude, high-frequency cycles
- Sequences during high-amplitude, high-frequency cycles
- Predicting carbonate reservoir location and quality
- Carbonate diagenesis
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Longman, M. W., 1980, Carbonate diagenetic textures from nearsurface diagenetic environments: AAPG Bulletin, vol. 64, no. 4, p. 461–487.
- ↑ Purser, B. H., 1978, Early diagenesis and the preservation of porosity in Jurassic limestones: Journal of Petroleum Geology, v. 1, no. 2, p. 83-94