Difference between revisions of "Ti in zircon geothermometer"
Cwhitehurst (talk | contribs) |
|||
| (21 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Wiki Write Off Entry | ||
| + | |image= AAPG-WIKI-Write-off.jpg | ||
| + | |student chapter= [http://geology.lsu.edu/GraduateStudents/CurrentStudents/AAPGStudentChapter/item33119.html Louisiana State University] | ||
| + | |competition= December 2014 | ||
| + | }} | ||
| + | '''Zircon Titanium Geothermometry''', or "Ti-in-zircon [[Geothermometry]]" is a technique by which the crystallization temperature of a [[zircon]] crystal can be estimated by the amount of titanium atoms found in the crystal lattice. Zircon is a useful mineral due to its abundance in the Earth's [[crust]], its ability to incorporate various [[radioactive]] and nonradioactive isotopes, its durability, and its resistance to isotopic diffusion.<ref name="Watson Wark Thomas 2006">Watson, E. B., D. A. Wark, & J. B. Thomas, 2006, Crystallization thermometers for zircon and rutile: Contributions to Mineral Petrology, vol. 151, pp. 413–433. doi:10.1007/s00410-006-0068-5</ref> In zircon crystals, [[Titanium]] is commonly incorporated, replacing similarly charged [[Zirconium]] and [[Silica]] atoms. This process is relatively unaffected by pressure and highly temperature dependent, with the amount of Ti incorporated rising exponentially with temperature,<ref name="Watson Wark Thomas 2006" /><ref name="Watson and Harrison 2005">Watson, E. B., & T. M. Harrison, 2005, Zircon Thermometer Reveals Minimum Melting Conditions on Earliest Earth: Science Magazine, vol. 308, pp. 841–843. doi:10.1126/science.1110873</ref> making this an accurate geothermometry method. This method is extremely useful, as it can be combined with radiometric dating techniques that are commonly used with zircon crystals (see [[Uranium-lead dating]]), to correlate quantitative temperature measurements with specific absolute ages. | ||
| + | |||
| + | ==Zircon== | ||
[[Image:zircon microscope.jpg|right|150px|thumb|Optical microscope photograph; the length of the crystal is about 250 [[µm]].]] | [[Image:zircon microscope.jpg|right|150px|thumb|Optical microscope photograph; the length of the crystal is about 250 [[µm]].]] | ||
| − | |||
| − | |||
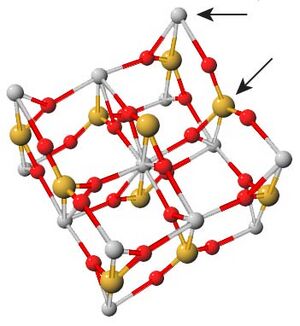
[[File:Titanium Substitutions in Zircon Unit Cell.jpeg|thumbnail|right|Image shows a unit cell of zircon. Arrows point to the possible substitution locations for titanium atoms. Yellow balls represent silicon atoms, grey balls represent zirconium atoms.]] | [[File:Titanium Substitutions in Zircon Unit Cell.jpeg|thumbnail|right|Image shows a unit cell of zircon. Arrows point to the possible substitution locations for titanium atoms. Yellow balls represent silicon atoms, grey balls represent zirconium atoms.]] | ||
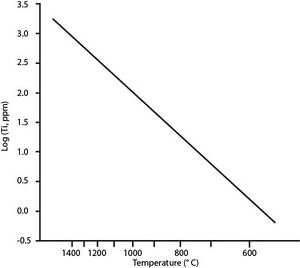
[[File:Log(Ti) vs T graph.jpeg|thumbnail|right|Plot of Ti abundance (log of Ti ppm) versus Temperature in Celsius. Simplified version, modified from Watson and Harrison 2005.]] | [[File:Log(Ti) vs T graph.jpeg|thumbnail|right|Plot of Ti abundance (log of Ti ppm) versus Temperature in Celsius. Simplified version, modified from Watson and Harrison 2005.]] | ||
Zircon ((Zr<sub>1–y</sub>, [[Rare earth elements|REE]]<sub>y</sub>)(SiO<sub>4</sub>)<sub>1–x</sub>(OH)<sub>4x–y</sub>)) is an [[orthosilicate]] mineral that is commonly found as an accessory mineral throughout Earth's crust.<ref name="Finch and Hanchar, structure of zircon" /> | Zircon ((Zr<sub>1–y</sub>, [[Rare earth elements|REE]]<sub>y</sub>)(SiO<sub>4</sub>)<sub>1–x</sub>(OH)<sub>4x–y</sub>)) is an [[orthosilicate]] mineral that is commonly found as an accessory mineral throughout Earth's crust.<ref name="Finch and Hanchar, structure of zircon" /> | ||
| − | Zircon is a particularly useful mineral because of its ability to incorporate many trace elements. It is know to exchange [[Rare Earth Elements]] (REE) such as [[Uranium]], [[Thorium]], [[Yttrium]],<ref name="Bea. Residence of REE, Y, Th and U..."> | + | Zircon is a particularly useful mineral because of its ability to incorporate many trace elements. It is know to exchange [[Rare Earth Elements]] (REE) such as [[Uranium]], [[Thorium]], [[Yttrium]],<ref name="Bea. Residence of REE, Y, Th and U...">Bea, F.,1996, Residence of REE, Y, Th and U in Granites and Crustal Protoliths; Implications for the Chemistry of Crustal Melts, Journal of Petrology 37 (3): 521-552.</ref> and [[Lutetium]]. However, the chemical potential energies of these REE substitutions are not well understood, so they are not suitable for determining crystallization temperatures. Titanium is also incorporated into zircon, and its exchange rates has been studied in detail. Ti<sup>4+</sup>, a tetravalent ion, can replace Zr<sup>4+</sup> or Si<sup>4+</sup> in a temperature dependent mechanism. For zircons in the presence of TiO<sub>2</sub>, i.e. the mineral [[rutile]], this substitution process is common and can be measured.<ref name="Watson Wark Thomas 2006" /> Zircon is also useful because its incorporation of other elements like Uranium, Lutetium, [[Samarium]],<ref name="Kinny and Maas Lu-Hf and Sm-Nd...">Kinny, Peter D., & R. Maas, 2003, Lu–Hf and Sm–Nd isotope systems in zircon: Reviews in Mineralogy and Geochemistry 53 (1): 327-341. doi:10.2113/0530327.</ref> and [[Oxygen]]<ref name="Valley Oxygen Isotopes in Zircon">Valley, J. W., 2003, Oxygen Isotopes in Zircon: Reviews in Mineralogy and Geochemistry 53 (1): 343-385. doi:10.2113/0530343.</ref> can be analyzed to provide further insight into the age and conditions the crystal grew under. |
| − | Thermally, zircon is resistant to temperature changes and extremes. It is stable up to 1690 °C at ambient pressure and has a low thermal expansion rate. Zircons crystals are also some of the most incompressible silicate minerals.<ref name="Finch and Hanchar, structure of zircon"> | + | Thermally, zircon is resistant to temperature changes and extremes. It is stable up to 1690 °C at ambient pressure and has a low thermal expansion rate. Zircons crystals are also some of the most incompressible silicate minerals.<ref name="Finch and Hanchar, structure of zircon">Finch, R. J., & J. M. Hanchar, 2003, Structure and chemistry of zircon and zircon-group minerals: Reviews in Mineralogy and Geochemistry 53 (1): 1–25. doi:10.2113/0530001</ref> The high durability of zircons also allows them to crystallize around other silicate minerals, creating pockets, or inclusions, of surrounding melts that are indicative of magma at specific pressures and temperatures. This essentially forms a time-capsule giving a glimpse of past conditions in which the crystal formed.<ref name="Thomas et al. Melt Inclusions in Zircon">Thomas, J.B., R. J. Bodnar, N. Shimizu, N., & C. A. Chesner, 2003, Melt Inclusions in Zircon: Reviews in Mineralogy and Geochemistry 53 (1): 63-87. doi:10.2113/0530063.</ref> |
| − | Zircons are know to be relatively retentive of their incorporated isotopes and thus very useful for microquantitative studies. Cations such as REE,<ref name="REE dif"> | + | Zircons are know to be relatively retentive of their incorporated isotopes and thus very useful for microquantitative studies. Cations such as REE,<ref name="REE dif">Cherniak, D. J., J. M. Hanchar, & E. B. Watson, Rare earth diffusion in zircon: Chemical Geology 134 (4): 289–301. doi:10.1016/S0009-2541(96)00098-8.</ref> U, Th, Hf,<ref name="Tetravalent diffusion">Cherniak, D. J., J. M. Hanchar, & E. B. Watson, Diffusion of tetravalent cations in zircon: Contributions to Mineralogy and Petrology 127 (4): 383–390. doi:10.1007/s004100050287.</ref> [[Pb]],<ref name="Pb diffusion">Cherniak, D. J., & E. B. Watson, Pb diffusion in zircon: Chemical Geology 172 (1-2): 5–24. doi:10.1016/S0009-2541(00)00233-3.</ref> and Ti<ref name="Cherniak and Watson 2007">Cherniak, D. J., & E. B. Watson, 2007, Ti diffusion in zircon: Chemical Geology 242: 470–483. doi:10.1016/j.chemgeo.2007.05.005.</ref> diffuse slowly out of zircons, and their measured quantities in the mineral are diagnostic of the melt conditions surrounding the crystal during growth. This slow rate of diffusion of many of the incorporated elements makes zircon crystals more likely to form compositional zoning, which may represent oscillatory zoning or sector zoning, as the melt composition or energy conditions change around the crystal over time.<ref name="Cherniak and Watson 2003, RMG">Cherniak, D. J., & E. B. Watson, 2003, Diffusion in Zircon". Reviews in Mineralogy and Geochemistry 53: 113–133. doi:10.2113/0530113.</ref> These zones show compositional differences between the core and rim of the crystal, providing observable evidence of changes in melt conditions.<ref name="Corfu et al. Atlas of Zircon Textures">Corfu, F., J. M. Hanchar, P. W. O. Hoskin, & P. Kinny, 2003, Atlas of Zircon Textures". Reviews in Mineralogy and Geochemistry 53 (1): 469-500. doi:10.2113/0530469. Retrieved 29 November 2014.</ref> Slow diffusion rates also prevent contamination by leaking or loss of isotopes from the crystal, increasing the likelihood that chonologic and compositional measurements are accurate. |
==Methods== | ==Methods== | ||
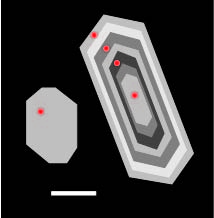
[[File:Cartoon zoned and unzoned zircon.jpeg|thumbnail|right|Cartoon version of unzoned (left) and zoned (right) zircons. Red dots represent ion microprobe scan locations. White bar is about 50 μm.]] | [[File:Cartoon zoned and unzoned zircon.jpeg|thumbnail|right|Cartoon version of unzoned (left) and zoned (right) zircons. Red dots represent ion microprobe scan locations. White bar is about 50 μm.]] | ||
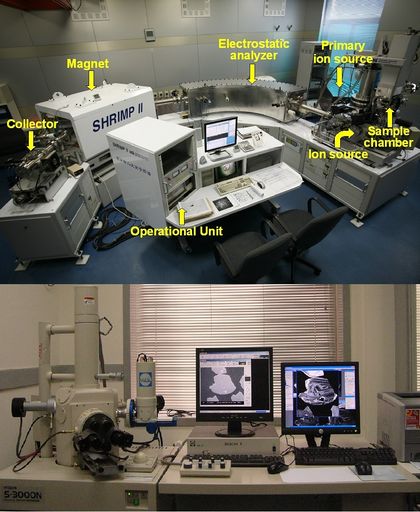
| − | [[File:Shrimp and SEM CL.jpeg|420px|thumbnail|right|Top: Shows a Sensitive High-Resolution Ion Microprobe (SHRIMP) at the Beijing Microprobe Center. This machine is commonly used to radiometrically date minerals using U/Pb, but can be used to measure Ti content of zircons. Bottom: Shows a Scanning Electron Microscope (SEM) equipped with Cathodoluminescence imaging at the Beijing Microprobe Center.<ref name=UMB> | + | [[File:Shrimp and SEM CL.jpeg|420px|thumbnail|right|Top: Shows a Sensitive High-Resolution Ion Microprobe (SHRIMP) at the Beijing Microprobe Center. This machine is commonly used to radiometrically date minerals using U/Pb, but can be used to measure Ti content of zircons. Bottom: Shows a Scanning Electron Microscope (SEM) equipped with Cathodoluminescence imaging at the Beijing Microprobe Center.<ref name=UMB>"[http://www.geo.unimib.it/SROS/beijing.html SHRIMP Remote Operations System (SROS)]". University of Milano-Bicocca. Retrieved 13 November 2014.</ref>]] |
| − | Once a rock has been collected, zircons are extracted using a series of techniques such as using a [[sieve]], [[heavy liquid]], shaking table, and [[magnetic separation]] to separate minerals based on differing densities and properties. Zircon crystals are then mounted to an epoxy or metal disc-shaped slide,<ref name="Microprobe prep"> | + | Once a rock has been collected, zircons are extracted using a series of techniques such as using a [[sieve]], [[heavy liquid]], shaking table, and [[magnetic separation]] to separate minerals based on differing densities and properties. Zircon crystals are then mounted to an epoxy or metal disc-shaped slide,<ref name="Microprobe prep"> "[http://shrimprg.stanford.edu/ Sample Preparation]". SHRIMP RG Sample Preparation. USGS. Retrieved 8 Oct 2014.</ref> where they can be shaved to about half thickness to reveal their internal structure. From here, they can be imaged using [[cathodoluminescence]] to make any zonations in the mineral visible. If zonation is apparent, multiple measurements of Ti abundance can be taken from the center to the rim to give the temperature evolution of the crystal. |
| − | The final step involves measuring the abundance of titanium in a specific location on a zircon crystal with an ion microprobe. For this, the chemical compositions of the zircons is measure using [[secondary ion mass spectrometry]]. The sample is bombarded with a beam of primary ions, and the charge and mass of the ejected secondary ions are measured to determine the chemical composition at the point of contact. The quantitative value for titanium content is then compared to a known relationship of titanium incorporation and temperature to determine the crystallization temperature of that zone of the zircon. The titanium-to-temperature relationship was calculated using in situ radiometrically dated zircons with known melt temperatures from the surrounding rock. This Ti-in-zircon measurement can be done several times in zoned zircons, which may record the temperature evolution that resulted from many geologic events.<ref name="Watson Wark Thomas 2006" /><ref name="Watson and Harrison 2005" /><ref name="hoskin and schaltegger, comp of zircon"> | + | The final step involves measuring the abundance of titanium in a specific location on a zircon crystal with an ion microprobe. For this, the chemical compositions of the zircons is measure using [[secondary ion mass spectrometry]]. The sample is bombarded with a beam of primary ions, and the charge and mass of the ejected secondary ions are measured to determine the chemical composition at the point of contact. The quantitative value for titanium content is then compared to a known relationship of titanium incorporation and temperature to determine the crystallization temperature of that zone of the zircon. The titanium-to-temperature relationship was calculated using in situ radiometrically dated zircons with known melt temperatures from the surrounding rock. This Ti-in-zircon measurement can be done several times in zoned zircons, which may record the temperature evolution that resulted from many geologic events.<ref name="Watson Wark Thomas 2006" /><ref name="Watson and Harrison 2005" /><ref name="hoskin and schaltegger, comp of zircon">Hoskin, P. W., & U. Schaltegger, The composition of zircon and igneous and metamorphic petrogenesis". Reviews in Mineralogy and Geochemistry 53 (1): 27–62. doi:10.2113/0530027.</ref> |
==Uses== | ==Uses== | ||
| Line 26: | Line 32: | ||
Ti-in-zircon geothermometry has so far been used in [[igneous]] rocks to estimate cooling temperatures of [[magma]] from zircon crystals dated to the [[Hadean]] age (>4.0 Ga). Low crystallization temperatures from zircons of this age suggests the Hadean Earth contained liquid water, which reduced the cooling temperature of crustal materials.<ref name="Watson and Harrison 2005" /> Future studies using Ti-in-zircon thermometry can show the progressive heat loss from a magmatic Hadean Earth, to the onset of [[plate tectonics]] as the planet's crust begins to cool and deform. This will provide previously unknown evidence for the conditions in early Earth and allow testing of ideas of how the planet evolved through the Hadean and [[Archean]] eons. | Ti-in-zircon geothermometry has so far been used in [[igneous]] rocks to estimate cooling temperatures of [[magma]] from zircon crystals dated to the [[Hadean]] age (>4.0 Ga). Low crystallization temperatures from zircons of this age suggests the Hadean Earth contained liquid water, which reduced the cooling temperature of crustal materials.<ref name="Watson and Harrison 2005" /> Future studies using Ti-in-zircon thermometry can show the progressive heat loss from a magmatic Hadean Earth, to the onset of [[plate tectonics]] as the planet's crust begins to cool and deform. This will provide previously unknown evidence for the conditions in early Earth and allow testing of ideas of how the planet evolved through the Hadean and [[Archean]] eons. | ||
| − | On a more recent timescale, Ti-in-zircon geothermometry can be used in zircons found in metamorphic rocks to estimate the pressure and temperatures conditions during metamorphosis. This helps identify the [[Metamorphic facies]] and thus the geologic setting of a rock formation.<ref | + | On a more recent timescale, Ti-in-zircon geothermometry can be used in zircons found in metamorphic rocks to estimate the pressure and temperatures conditions during metamorphosis. This helps identify the [[Metamorphic facies]] and thus the geologic setting of a rock formation.<ref>Ewing, T. A., J. Hermann, & D. Rubatto, 2013, The robustness of the Zr-in-rutile and Ti-in-zircon thermometers during high-temperature metamorphism (Ivrea-Verbano Zone, northern Italy): Contributions to Mineralogy and Petrology 165 (4): 757-779. doi:10.1007/s00410-012-0834-5.</ref><ref name="Liu et al. 2015 Application of Ti-in-zircon on Dabie orogen">Liu, Y.-C., L.-P. Deng, X.-F. Gu, C. Groppo, & F. Rolfo, 2015, Application of Ti-in-zircon and Zr-in-rutile thermometers to constrain high-temperature metamorphism in eclogites from the Dabie orogen, central China: Gondwana Research 27 (1): 410-423. doi:10.1016/j.gr.2013.10.011.</ref> It can also be used in sedimentary rocks to help determine the source of [[detrital]] minerals. However, these crystals may sometimes be contaminated by external titanium seeping into fractures.<ref name="Watson Wark Thomas 2006" /><ref name="hoskin and schaltegger, comp of zircon" /> |
==Errors and Limitations== | ==Errors and Limitations== | ||
| − | Ti-in-Zircon geothermometry is considered to be a relatively reliable and accurate method of determining crystallization temperatures of zircons, with a error of only 10-16 degrees Celsius. However, the major constraint of this process is that it is only usable in systems that contain titanium, or the mineral [[rutile]] (TiO<sub>2</sub>). In systems that have no or very little titanium, this method is pointless, as zircons will not incorporate titanium if it is not present in the magmatic melt.<ref name="Watson and Harrison 2005" /> However, recent models have taken into account zircon's ability to replace either silicon or zirconium in the crystal with titanium by using independent activities of Si and Zr.<ref name="Ferry and Watson 2007 New Thermo models and revised calibrations"> | + | Ti-in-Zircon geothermometry is considered to be a relatively reliable and accurate method of determining crystallization temperatures of zircons, with a error of only 10-16 degrees Celsius. However, the major constraint of this process is that it is only usable in systems that contain titanium, or the mineral [[rutile]] (TiO<sub>2</sub>). In systems that have no or very little titanium, this method is pointless, as zircons will not incorporate titanium if it is not present in the magmatic melt.<ref name="Watson and Harrison 2005" /> However, recent models have taken into account zircon's ability to replace either silicon or zirconium in the crystal with titanium by using independent activities of Si and Zr.<ref name="Ferry and Watson 2007 New Thermo models and revised calibrations">Ferry, J. M., & E. B. Watson, 2007, New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers: Contributions to Mineralogy and Petrology 154 (4): 429-437. doi:10.1007/s00410-007-0201-0.</ref> This has expanded the potential uses for zircons with unknown origins, due to the abundance of silicon in Earth's crust. In some zircon crystals, inclusions of the mineral [[quartz]] (SiO<sub>2</sub>) can be used as proof that silicon was present during crystallization, thus validating the use of this geothermometer. |
Due to the abundance of radioactive elements that can by incorporated into zircons, they are also susceptible to damage from radioactive decay through the process of [[Metamictization]]. As radioactive elements inside the crystal lattice decay, they bombard the interior of the crystal with radioactive particles. This weakens the crystal and leave it fractured or weakened.<ref name="hoskin and schaltegger, comp of zircon" /> This increases the chance of isotopes leaking out of the crystal and affecting titanium, or other elements, measurements. | Due to the abundance of radioactive elements that can by incorporated into zircons, they are also susceptible to damage from radioactive decay through the process of [[Metamictization]]. As radioactive elements inside the crystal lattice decay, they bombard the interior of the crystal with radioactive particles. This weakens the crystal and leave it fractured or weakened.<ref name="hoskin and schaltegger, comp of zircon" /> This increases the chance of isotopes leaking out of the crystal and affecting titanium, or other elements, measurements. | ||
| Line 35: | Line 41: | ||
Another difficulty with this microanalysis is the contamination of Ti on external surfaces. Recent studies have expressed concern over the [[gold]] coating on the surface of the ion microprobe mounts, which contains small amounts of Ti (~1 ppm) that could provide an error during measurement. In [[detrital]] zircons found in sedimentary sources, a Ti-bearing oxide coating on the surface and in fractures of zircons can also contaminate the crystal with excess titanium.<ref name="Watson Wark Thomas 2006" /> | Another difficulty with this microanalysis is the contamination of Ti on external surfaces. Recent studies have expressed concern over the [[gold]] coating on the surface of the ion microprobe mounts, which contains small amounts of Ti (~1 ppm) that could provide an error during measurement. In [[detrital]] zircons found in sedimentary sources, a Ti-bearing oxide coating on the surface and in fractures of zircons can also contaminate the crystal with excess titanium.<ref name="Watson Wark Thomas 2006" /> | ||
| − | More recent studies have also shown that there are additional unknown factors that contribute to Ti incorporation in zircons. The chemical activity of SiO<sub>2</sub>, pressure variance, disequilibrium crystallization from melts, late crystal growth in hydrous melts, or non-[[Henry's Law]] substitution in zircon crystals all may play a role in altering predicted crystallization temperatures.<ref name="Fu et al, applications and limitations"> | + | More recent studies have also shown that there are additional unknown factors that contribute to Ti incorporation in zircons. The chemical activity of SiO<sub>2</sub>, pressure variance, disequilibrium crystallization from melts, late crystal growth in hydrous melts, or non-[[Henry's Law]] substitution in zircon crystals all may play a role in altering predicted crystallization temperatures.<ref name="Fu et al, applications and limitations">Fu, B., F. Z. Page, A. J. Cavosie, J. Fournelle, N. T. Kita, T. Noriko, J. S. Lackey, S. A. Wilde, & J. W. Valey, 2008, Ti-in-zircon thermometry: applications and limitations: Contributions to Mineralogy and Petrology 156: 197–215. doi:10.1007/s00410-008-0281-5.</ref> |
This technique is also constrained by several assumptions that, while valid, my prove inconsistent in certain situations. Laboratory studies have used constant pressures when calculating cooling temperatures and have assumed that pressure does not play a major role in Ti incorporation. When estimating cooling temperatures, increased pressure is accounted for by increased temperature estimates and thus increases the uncertainty of the estimates.<ref name="Ferry and Watson 2007 New Thermo models and revised calibrations" /> | This technique is also constrained by several assumptions that, while valid, my prove inconsistent in certain situations. Laboratory studies have used constant pressures when calculating cooling temperatures and have assumed that pressure does not play a major role in Ti incorporation. When estimating cooling temperatures, increased pressure is accounted for by increased temperature estimates and thus increases the uncertainty of the estimates.<ref name="Ferry and Watson 2007 New Thermo models and revised calibrations" /> | ||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
Latest revision as of 22:11, 3 December 2015
| Wiki Write-Off Entry | |
|---|---|

| |
| Student Chapter | Louisiana State University |
| Competition | December 2014 |
Zircon Titanium Geothermometry, or "Ti-in-zircon Geothermometry" is a technique by which the crystallization temperature of a zircon crystal can be estimated by the amount of titanium atoms found in the crystal lattice. Zircon is a useful mineral due to its abundance in the Earth's crust, its ability to incorporate various radioactive and nonradioactive isotopes, its durability, and its resistance to isotopic diffusion.[1] In zircon crystals, Titanium is commonly incorporated, replacing similarly charged Zirconium and Silica atoms. This process is relatively unaffected by pressure and highly temperature dependent, with the amount of Ti incorporated rising exponentially with temperature,[1][2] making this an accurate geothermometry method. This method is extremely useful, as it can be combined with radiometric dating techniques that are commonly used with zircon crystals (see Uranium-lead dating), to correlate quantitative temperature measurements with specific absolute ages.
Zircon[edit]

Zircon ((Zr1–y, REEy)(SiO4)1–x(OH)4x–y)) is an orthosilicate mineral that is commonly found as an accessory mineral throughout Earth's crust.[3]
Zircon is a particularly useful mineral because of its ability to incorporate many trace elements. It is know to exchange Rare Earth Elements (REE) such as Uranium, Thorium, Yttrium,[4] and Lutetium. However, the chemical potential energies of these REE substitutions are not well understood, so they are not suitable for determining crystallization temperatures. Titanium is also incorporated into zircon, and its exchange rates has been studied in detail. Ti4+, a tetravalent ion, can replace Zr4+ or Si4+ in a temperature dependent mechanism. For zircons in the presence of TiO2, i.e. the mineral rutile, this substitution process is common and can be measured.[1] Zircon is also useful because its incorporation of other elements like Uranium, Lutetium, Samarium,[5] and Oxygen[6] can be analyzed to provide further insight into the age and conditions the crystal grew under.
Thermally, zircon is resistant to temperature changes and extremes. It is stable up to 1690 °C at ambient pressure and has a low thermal expansion rate. Zircons crystals are also some of the most incompressible silicate minerals.[3] The high durability of zircons also allows them to crystallize around other silicate minerals, creating pockets, or inclusions, of surrounding melts that are indicative of magma at specific pressures and temperatures. This essentially forms a time-capsule giving a glimpse of past conditions in which the crystal formed.[7]
Zircons are know to be relatively retentive of their incorporated isotopes and thus very useful for microquantitative studies. Cations such as REE,[8] U, Th, Hf,[9] Pb,[10] and Ti[11] diffuse slowly out of zircons, and their measured quantities in the mineral are diagnostic of the melt conditions surrounding the crystal during growth. This slow rate of diffusion of many of the incorporated elements makes zircon crystals more likely to form compositional zoning, which may represent oscillatory zoning or sector zoning, as the melt composition or energy conditions change around the crystal over time.[12] These zones show compositional differences between the core and rim of the crystal, providing observable evidence of changes in melt conditions.[13] Slow diffusion rates also prevent contamination by leaking or loss of isotopes from the crystal, increasing the likelihood that chonologic and compositional measurements are accurate.
Methods[edit]
Once a rock has been collected, zircons are extracted using a series of techniques such as using a sieve, heavy liquid, shaking table, and magnetic separation to separate minerals based on differing densities and properties. Zircon crystals are then mounted to an epoxy or metal disc-shaped slide,[15] where they can be shaved to about half thickness to reveal their internal structure. From here, they can be imaged using cathodoluminescence to make any zonations in the mineral visible. If zonation is apparent, multiple measurements of Ti abundance can be taken from the center to the rim to give the temperature evolution of the crystal.
The final step involves measuring the abundance of titanium in a specific location on a zircon crystal with an ion microprobe. For this, the chemical compositions of the zircons is measure using secondary ion mass spectrometry. The sample is bombarded with a beam of primary ions, and the charge and mass of the ejected secondary ions are measured to determine the chemical composition at the point of contact. The quantitative value for titanium content is then compared to a known relationship of titanium incorporation and temperature to determine the crystallization temperature of that zone of the zircon. The titanium-to-temperature relationship was calculated using in situ radiometrically dated zircons with known melt temperatures from the surrounding rock. This Ti-in-zircon measurement can be done several times in zoned zircons, which may record the temperature evolution that resulted from many geologic events.[1][2][16]
Uses[edit]
Geothermometry techniques can provide evidence for changes in temperature in various environments, the thermodynamic evolution of rocks, the gradual change in the geothermal gradient over geologic time, and determine provenance of detrital sediments. Coupled with geochronology techniques using radiometric decay and dating, such as with U/Pb decay, these paleotemperature measurements can be paired with an absolute age in order to determine temperature changes over time.
Ti-in-zircon geothermometry has so far been used in igneous rocks to estimate cooling temperatures of magma from zircon crystals dated to the Hadean age (>4.0 Ga). Low crystallization temperatures from zircons of this age suggests the Hadean Earth contained liquid water, which reduced the cooling temperature of crustal materials.[2] Future studies using Ti-in-zircon thermometry can show the progressive heat loss from a magmatic Hadean Earth, to the onset of plate tectonics as the planet's crust begins to cool and deform. This will provide previously unknown evidence for the conditions in early Earth and allow testing of ideas of how the planet evolved through the Hadean and Archean eons.
On a more recent timescale, Ti-in-zircon geothermometry can be used in zircons found in metamorphic rocks to estimate the pressure and temperatures conditions during metamorphosis. This helps identify the Metamorphic facies and thus the geologic setting of a rock formation.[17][18] It can also be used in sedimentary rocks to help determine the source of detrital minerals. However, these crystals may sometimes be contaminated by external titanium seeping into fractures.[1][16]
Errors and Limitations[edit]
Ti-in-Zircon geothermometry is considered to be a relatively reliable and accurate method of determining crystallization temperatures of zircons, with a error of only 10-16 degrees Celsius. However, the major constraint of this process is that it is only usable in systems that contain titanium, or the mineral rutile (TiO2). In systems that have no or very little titanium, this method is pointless, as zircons will not incorporate titanium if it is not present in the magmatic melt.[2] However, recent models have taken into account zircon's ability to replace either silicon or zirconium in the crystal with titanium by using independent activities of Si and Zr.[19] This has expanded the potential uses for zircons with unknown origins, due to the abundance of silicon in Earth's crust. In some zircon crystals, inclusions of the mineral quartz (SiO2) can be used as proof that silicon was present during crystallization, thus validating the use of this geothermometer.
Due to the abundance of radioactive elements that can by incorporated into zircons, they are also susceptible to damage from radioactive decay through the process of Metamictization. As radioactive elements inside the crystal lattice decay, they bombard the interior of the crystal with radioactive particles. This weakens the crystal and leave it fractured or weakened.[16] This increases the chance of isotopes leaking out of the crystal and affecting titanium, or other elements, measurements.
Another difficulty with this microanalysis is the contamination of Ti on external surfaces. Recent studies have expressed concern over the gold coating on the surface of the ion microprobe mounts, which contains small amounts of Ti (~1 ppm) that could provide an error during measurement. In detrital zircons found in sedimentary sources, a Ti-bearing oxide coating on the surface and in fractures of zircons can also contaminate the crystal with excess titanium.[1]
More recent studies have also shown that there are additional unknown factors that contribute to Ti incorporation in zircons. The chemical activity of SiO2, pressure variance, disequilibrium crystallization from melts, late crystal growth in hydrous melts, or non-Henry's Law substitution in zircon crystals all may play a role in altering predicted crystallization temperatures.[20]
This technique is also constrained by several assumptions that, while valid, my prove inconsistent in certain situations. Laboratory studies have used constant pressures when calculating cooling temperatures and have assumed that pressure does not play a major role in Ti incorporation. When estimating cooling temperatures, increased pressure is accounted for by increased temperature estimates and thus increases the uncertainty of the estimates.[19]
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Watson, E. B., D. A. Wark, & J. B. Thomas, 2006, Crystallization thermometers for zircon and rutile: Contributions to Mineral Petrology, vol. 151, pp. 413–433. doi:10.1007/s00410-006-0068-5
- ↑ 2.0 2.1 2.2 2.3 Watson, E. B., & T. M. Harrison, 2005, Zircon Thermometer Reveals Minimum Melting Conditions on Earliest Earth: Science Magazine, vol. 308, pp. 841–843. doi:10.1126/science.1110873
- ↑ 3.0 3.1 Finch, R. J., & J. M. Hanchar, 2003, Structure and chemistry of zircon and zircon-group minerals: Reviews in Mineralogy and Geochemistry 53 (1): 1–25. doi:10.2113/0530001
- ↑ Bea, F.,1996, Residence of REE, Y, Th and U in Granites and Crustal Protoliths; Implications for the Chemistry of Crustal Melts, Journal of Petrology 37 (3): 521-552.
- ↑ Kinny, Peter D., & R. Maas, 2003, Lu–Hf and Sm–Nd isotope systems in zircon: Reviews in Mineralogy and Geochemistry 53 (1): 327-341. doi:10.2113/0530327.
- ↑ Valley, J. W., 2003, Oxygen Isotopes in Zircon: Reviews in Mineralogy and Geochemistry 53 (1): 343-385. doi:10.2113/0530343.
- ↑ Thomas, J.B., R. J. Bodnar, N. Shimizu, N., & C. A. Chesner, 2003, Melt Inclusions in Zircon: Reviews in Mineralogy and Geochemistry 53 (1): 63-87. doi:10.2113/0530063.
- ↑ Cherniak, D. J., J. M. Hanchar, & E. B. Watson, Rare earth diffusion in zircon: Chemical Geology 134 (4): 289–301. doi:10.1016/S0009-2541(96)00098-8.
- ↑ Cherniak, D. J., J. M. Hanchar, & E. B. Watson, Diffusion of tetravalent cations in zircon: Contributions to Mineralogy and Petrology 127 (4): 383–390. doi:10.1007/s004100050287.
- ↑ Cherniak, D. J., & E. B. Watson, Pb diffusion in zircon: Chemical Geology 172 (1-2): 5–24. doi:10.1016/S0009-2541(00)00233-3.
- ↑ Cherniak, D. J., & E. B. Watson, 2007, Ti diffusion in zircon: Chemical Geology 242: 470–483. doi:10.1016/j.chemgeo.2007.05.005.
- ↑ Cherniak, D. J., & E. B. Watson, 2003, Diffusion in Zircon". Reviews in Mineralogy and Geochemistry 53: 113–133. doi:10.2113/0530113.
- ↑ Corfu, F., J. M. Hanchar, P. W. O. Hoskin, & P. Kinny, 2003, Atlas of Zircon Textures". Reviews in Mineralogy and Geochemistry 53 (1): 469-500. doi:10.2113/0530469. Retrieved 29 November 2014.
- ↑ "SHRIMP Remote Operations System (SROS)". University of Milano-Bicocca. Retrieved 13 November 2014.
- ↑ "Sample Preparation". SHRIMP RG Sample Preparation. USGS. Retrieved 8 Oct 2014.
- ↑ 16.0 16.1 16.2 Hoskin, P. W., & U. Schaltegger, The composition of zircon and igneous and metamorphic petrogenesis". Reviews in Mineralogy and Geochemistry 53 (1): 27–62. doi:10.2113/0530027.
- ↑ Ewing, T. A., J. Hermann, & D. Rubatto, 2013, The robustness of the Zr-in-rutile and Ti-in-zircon thermometers during high-temperature metamorphism (Ivrea-Verbano Zone, northern Italy): Contributions to Mineralogy and Petrology 165 (4): 757-779. doi:10.1007/s00410-012-0834-5.
- ↑ Liu, Y.-C., L.-P. Deng, X.-F. Gu, C. Groppo, & F. Rolfo, 2015, Application of Ti-in-zircon and Zr-in-rutile thermometers to constrain high-temperature metamorphism in eclogites from the Dabie orogen, central China: Gondwana Research 27 (1): 410-423. doi:10.1016/j.gr.2013.10.011.
- ↑ 19.0 19.1 Ferry, J. M., & E. B. Watson, 2007, New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers: Contributions to Mineralogy and Petrology 154 (4): 429-437. doi:10.1007/s00410-007-0201-0.
- ↑ Fu, B., F. Z. Page, A. J. Cavosie, J. Fournelle, N. T. Kita, T. Noriko, J. S. Lackey, S. A. Wilde, & J. W. Valey, 2008, Ti-in-zircon thermometry: applications and limitations: Contributions to Mineralogy and Petrology 156: 197–215. doi:10.1007/s00410-008-0281-5.