Quick-look lithology from logs
| It has been suggested that some portions of this article be split into multiple articles. |
Lithological determination from wireline logs is often done by sophisticated computer programs, but basic quick-look interpretation can be made by visual inspection of appropriate logs.
The best logs for lithological purposes are those that are (1) most influenced by rock properties and (2) least influenced by fluid properties. The most useful of the commonly available logs are
- Gamma ray
- Spontaneous potential (SP)
- Caliper
- Formation density
- Photoelectric absorption
- Neutron porosity
(For more details on these logs, see Basic open hole tools. Also, Difficult lithologies covers logging tool response in sedimentary minerals.)
Borehole imaging tools such as the Formation MicroScanner are invaluable for detailed purposes, including bedding character and sedimentary structures, but are much less commonly available.
Gamma ray logs[edit]
The common radioactive elements—potassium, thorium, and uranium—are normally insignificant in reservoir fluids, whereas they are important components of the rock system, especially of clay minerals. Gamma ray logs are therefore a good indicator of mineralogy.
Lithological responses[edit]
The principal gamma ray responses are as follows:
| Lithology | Gamma Ray Values (in API units) |
|---|---|
| Sandstone (quartz) | 15–30 (rarely to 200) |
| Limestone | 10–40 |
| Dolomite | 15–40 (rarely to 200) |
| Shale | 60–150 |
| Organic-rich shale | 100–250 |
| Anhydrite, halite | 8–15 |
| Sylvite (KCI) | 350–500 |
| Coal | 15–150 (any value possible) |
Log shapes[edit]
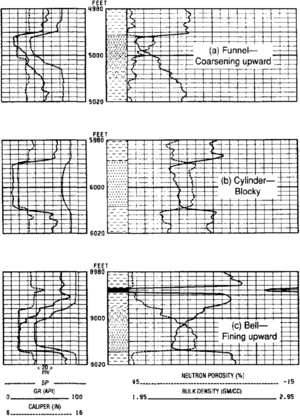
The shape of a gamma ray (or SP) log through a sand body is often thought of as a grain size profile. Three basic log shapes are recognized: funnel (coarsening upward), cylinder (blocky), and bell (fining upward) (Figure 1). These three shapes can be subdivided into smooth (relatively homogeneous) or serrate (with interbedded thin shales).
Log shapes typically reflect changing depositional energy from high (clean, coarser sand) to low (shaly, finer sand). An interpretive jump is usually made from depositional energy to depositional process and hence depositional environment. Often this jump is made without seriously considering the intermediate steps. This can be dangerous. Each of the steps is highly ambiguous and must be augmented by other evidence, such as unit thickness, associated rock types, and overall depositional setting. Typically,
- Funnel shapes imply upward-increasing energy, which may be found in distributary mouth bars, delta lobe fringes, deep sea fans, and other environments.
- Cylinder shapes reflect relatively constant energy levels and can include eolian dunes, low sinuosity distributary channels, and beaches.
- Bell shapes represent waning-current sequences, which can include alluvial point bars, deltaic distributaries, and deep sea fan channels.
In fact, grain size has no effect on gamma ray logs. The log shapes reflect shaliness, that is, clay and mica content of the sand. Because most sands reflect a hydrodynamic equilibrium, clay content does usually correlate (inversely) with grain size. However, in the following examples, clay content and grain size do not correlate, resulting in misleading log shapes:
- Very fine, clean sand above coarser sand may show a cylinder shape.
- Clay clasts concentrated near the base of a channel may give a funnel shape.
- Clay added later due to bioturbation or mechanical infiltration at the top of a gravel may create a bell shape.
(For more details on using log shape to interpret depositional environment, see Lithofacies and environmental analysis of clastic depositional systems.)
Problems and exceptions[edit]
- Radioactive minerals in sands, especially K-feldspar, zircon, and mica, can raise sand readings as high as adjacent shales. Gamma ray logs may be useless in immature sands derived from basement terranes. However, beach placers rich in zircon may be valuable correlative markers if not mistaken for shale.
- “Hot” dolomite, especially common in the Permian basin in the United States, may have gamma ray values up to 200 API units, resembling shale.
- Radioactive (KCl) muds raise the baseline gamma ray zero reading so that apparent values for all rock types are increased, sometimes by about 20 API units.
- Evanescent high gamma ray readings in sands, present on one logging run but vanished some weeks later, have been observed especially in steamflood conditions. While remaining enigmatic, these may be due to concentrations of radon in the pore space.
Spectral gamma ray logs[edit]
In this enhancement to natural gamma ray logging, the energy levels of incoming gamma rays are counted in a series of energy windows, and an algorithm converts the energy spectrum to count rates for potassium (%), thorium (ppm), and uranium (ppm). Spectral gamma ray logs are most useful in identifying the following:
- Clay minerals. Illite clays are rich in potassium, whereas smectite and kaolinite contain thorium. The thorium to potassium ratio can distinguish illitic from smectitic shales and so provide a correlation tool.
- Organic-rich rocks. In shales, uranium enrichment is usually associated with organic content and can be a tool for identifying oil source beds. Quantitative relationships between uranium and organic content have been reported, but tend to be inconsistent.
- Mica sand. Richly micaceous sands (such as the Rannoch unit of the Brent Sand in the North Sea) appear shaly on gamma ray logs, but can be distinguished because the radiation is all from potassium.
- “Hot” dolomite. This type of dolomite can be distinguished from shale because the gamma rays are principally from uranium. The chemical relationship between uranium and the dolomite is unknown.
- Natural fractures. Soluble uranium in pore water often precipitates on open fractures, so thin intervals with high uranium count (a “spiky” log) may mark a fractured interval.
- Producing zones. As with natural fractures, uranium may precipitate on flowing perforations, so a spectral gamma ray log run after years of production may show which completed intervals are producing and which are not.
- Uranium prospecting. Most of the “uranium” signal actually comes from the tenth decay process in the uranium series, the decay of bismuth-214. This is separated in time from the original uranium by half-lives in excess of 109 years, so the relatively soluble uranium may have moved away during the interim even though the log still records its presence.
Spontaneous potential (SP) logs[edit]
Lithological responses[edit]
Shale[edit]
Spontaneous potential interpretation depends on first recognizing shale, where fairly constant SP readings form a straight “shale baseline” on the log (Figure 1a). Its actual SP value is not significant.
Sandstone[edit]
The potential differences around a sand-shale contact deflect the SP from the shale baseline. The deflection is negative for a normal salinity contrast (borehole fresher than formation). Little change occurs within a sand interval, so a clean sand shows a straight-line “sand line” (Figure 1c). (For more details on SP shale and sand baselines, see Determination of water resistivity.)
Tight rocks[edit]
An SP log is of little use in the absence of boundaries between shale beds and permeable beds. In relatively tight rocks (carbonates, evaporites, etc.), the SP wanders aimlessly, with no sharp usable deflections.
Log shapes[edit]
Funnel, cylinder, and bell-shaped motifs resemble those previously described for gamma ray logs. They are due to the qualitative shaliness indication given by the SP and can therefore be interpreted in a similar way to the gamma ray (except for the following complications).
Salinity contrast[edit]
Contrasting salinity is critical for SP logs. Three scenarios are possible:
- Fresh borehole fluid in a saline formation. Gives “normal” SP.
- Borehole salinity is same as formation. Featureless SP, very low amplitude, may be a straight line, no obvious relationship to beds (Figure 1b).
- Saline borehole in a fresher formation. Gives a reversed SP, where sands show positive deflections from the shale baseline.
Other problems[edit]
In additional to salinity contrasts, other conditions can create problems in interpreting SP logs. For example,
- Baseline shifts. Although the value of the SP shale baseline is not significant, it will shift if formation fluid salinity changes from one bed to another, making the log hard to interpret.
- Manual shifts. On occasion, the logging engineer adjusts the SP log scale to keep it within the track.
- Mud type. Water-based mud (with suitable salinity) is essential. Oil-filled or empty holes have nothing to carry the SP charges.
- Interference. Remanent magnetism within the winching system often ruins SP logs. Look for a sine-form SP whose cycle length is the circumference of the cable drum.
- Hydrocarbons. The SP is generated in water. High hydrocarbon saturation reduces the SP, making sands appear more shaly.
Caliper logs[edit]
Property measured[edit]
For lithological purposes, the critical data are caliper readings relative to bit size. There are three scenarios:
| Hard, inert rock | Hole in gauge | Caliper = bit size |
| Soft or brittle rock | Hole washes out | Caliper > bit size |
| Permeable rock | Mudcake builds up | Caliper |
Well-designed modern mud systems can minimize washouts, making caliper logs less distinctive for lithological purposes.
Lithological responses[edit]
Sandstone[edit]
Consolidated sandstone is usually permeable, so expect mudcake to cause a caliper reading that is about length::0.5 in. smaller than the bit size. Bed boundaries are often accurately delimited (Figure 1).
Sand[edit]
Friable, unconsolidated sand may wash out, causing large caliper readings. Look for this problem in young, shallow formations.
Shale[edit]
Shale frequently spalls into the borehole, especially in the minimum principal stress direction. This leads to elliptical boreholes identifiable with multiple arm calipers, as on a dipmeter.
Coal[edit]
Medium to high rank coals are often brittle and well-jointed. Such joint blocks cave into the borehole (Figure 1c) leaving deep washouts as thick as the coal seam (frequently only length::1 ft or so). Not all coals behave this way.
Carbonates[edit]
Carbonates often fail to show mudcake build-up despite good permeability because individual vuggy or moldic pores are too large to trap mud solids. Mudcake builds up on the back walls of such pores, not into the borehole. Sucrosic dolomite is the only carbonate that typically shows mudcake on calipers.
Tight rocks[edit]
Tightly cemented beds, such as ironstones, siltstones, and carbonate concretions in sandstones, are hard, inert rocks that remain in gauge.
Anhydrite and gypsum[edit]
Anhydrite and gypsum frequently remain in gauge if pure, but shaly intervals may be washed out.
Halite and potash salts[edit]
Salt-saturated or oil-based muds may maintain the hole in gauge, but dilute water-based muds result in severe dissolution leading to huge, unoriented washouts.
Formation density logs (Alone)[edit]
Property measured[edit]
Measured density is the sum of the rock system density and the pore fluid system density. Density values can therefore be used directly to identify lithology only when the porosity is insignificant. In porous rocks, density must be interpreted in combination with neutron or other porosity logs.
Lithological Responses (Nonporous rocks)[edit]
Evaporites[edit]
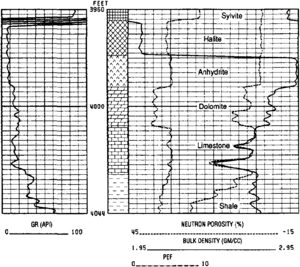
Individual evaporitic minerals (such as anhydrite, halite, sylvite, and carnallite) have well-defined densities and generate straight-line density logs with little variation (Figure 2).
Coal[edit]
Coals are variable but always significantly lighter than 2 g/cm3. Thin beds give a pronounced density spike, but may not resolve a true density reading (Figure 1c). Note that deep washouts also give low-density spikes.
Ironstone[edit]
Concentrations of iron minerals such as pyrite and siderite give high densities, often in thin beds, contrasting with surrounding rocks.
Shale[edit]
Densities of shales vary between 2.2 and 2.65 g/cm3 or more, increasing with compaction induced by age and depth of burial (Figure 1). Overpressured shales, in which some of the overburden load is borne by pore fluid, are undercompacted and have low densities relative to normally pressured shales at similar depths.
Photoelectric absorption (Pe) logs property measured[edit]
Photoelectric absorption (Pe), measured by the newer formation density tools, is related to atomic number Z, raised to the 3.6 power (Z3.6). Consequently, very light components (pore fluids) have negligible effect, making the log good for lithology. Unfortunately, heavy elements have an enormous effect. Thus, a few percent of iron masks basic lithological differences, and barite (usually with mud weights over 10 ppg) makes the log unusable.
Lithological responses[edit]
Sandstone[edit]
Quartz should read 1.7 to 1.8 barns/electron, but most other minerals can raise the value substantially. Because they are usually present, the log is of limited value.
Limestone[edit]
Clean limestone reads about 5.0 barns/electron (Figure 2).
Dolomite[edit]
Dolomite should read about 3.0 barns/electron, providing an easy way to distinguish limestone from dolomite (Figure 2) even if gas is present. Note that iron in ferroan dolomite increases readings to resemble limestone.
Shale[edit]
“Average” shale reads 3–3.5 barns/electron, but values up to 7 or 8 barns/electron can be obtained depending on iron content and accessory minerals. This large range makes the log of limited value.
Neutron porosity logs (Alone)[edit]
Property measured[edit]
Compensated neutron porosity is primarily the combined hydrogen content of the rock system and the pore fluid system. Lithology can therefore be interpreted directly from neutron values only when porosity is insignificant. In porous rocks, the neutron log must be interpreted in combination with other logs such as formation density.
Lithological responses (Nonporous Rocks)[edit]
Water of Crystallization (Evaporites)[edit]
- Gypsum and anhydrite. The typical neutron porosity value in anhydrite (CaSO4) is close to zero, but that in gypsum (CaSO4 • 2H2O) is much higher—up to 60%.
- Potash Evaporites. Sylvite is anhydrous with a near-zero neutron porosity, but carnallite (KMgCl3 • 6H2O) gives neutron values of 30% to 60%.
Bound water in shale[edit]
Some water in shales is chemically bound to clay minerals, whereas some occurs in micropores. Both types raise neutron log readings but represent no effective porosity (Figure 1). Shales consequently have high apparent neutron porosity, but values vary among formations. Often 40% is a good shale cutoff limit, but shale values can be as low as 30%. A local cut-off can often be established by calibration, such as from cores.
Neutron and density logs combined[edit]
Neutron and density logs each react to both lithology and porosity, so by analyzing the two logs together, one can begin to distinguish lithology from porosity. Neutron and density logs, together with a caliper measurement recorded by the density tool and a natural gamma ray log, are commonly run as a combination. This is the most powerful of the commonly available log suites for general purpose determination of lithology.
Crossplotting[edit]
Logging company chart books all include neutron-density crossplots that are easy to use for clean (nonshaly) reservoir rocks. The plots are entered with a bulk density and an apparent neutron porosity (should be environmentally corrected, but the corrections are usually negligible). A rock type (sandstone, limestone, or dolomite) and a corrected porosity can be read from the crossplot.
Overlay presentation[edit]
Manual crossplotting is tedious. A much faster way to visualize rock type is directly from the overlay presentation in which both neutron and density logs are superimposed in the same log track. To do this, a compatible scale must be used so that the porosity components of both logs exactly overlay. Then any offset (or residual) between the two logs is attributable to lithology or to the presence of gas.
Both tools are generally calibrated in limestone units, so the compatible scale is defined for freshwater-limestone systems, with theoretical limits as follows:
| All Porosity (H2O) | No Porosity (CaCO3) | |
|---|---|---|
| Neutron (p.u.) | 100 | 0 |
| Density (g/cm3 ) | 1.0 | 2.71 |
In practice, porosities over 50% are seldom needed, whereas rocks with densities over 2.71 g/cm3 are common. Thus, with slight rounding, the usual compatible scale is
| Neutron (p.u.) | 45 | 30 | 15 | 0 | –15 |
| Density (g/cm3 ) | 1.95 | 2.20 | 2.45 | 2.70 | 2.95 |
In high porosity areas with no dolomite, the scale is often slid across to the following range:
| Neutron (p.u.) | 60 | 45 | 30 | 15 | 0 |
| Density (g/cm3 ) | 1.70 | 1.95 | 2.20 | 2.45 | 2.70 |
On these scales, any offset of neutron and density logs is maintained regardless of porosity. Offsets are due to rock differences in density and neutron-absorbing properties (capture cross section). Ideal relationships for the three main liquid-filled porous rocks are as follows:
Sandstone[edit]
- Density displaced 0.05 g/cm3 to the left.
- Neutron displaced about 3 p.u. (porosity units) to the right.
- Cross-over is two small-scale divisions on the usual log grid.
Limestone[edit]
- Density and neutron overlay exactly.
Dolomite[edit]
- Density displaced 0.175 g/cm3 to the right.
- Neutron displaced 4–8 p.u. to the left.
- Separation is four to six small-scale divisions on the usual log grid.
Other noncompatible scales are harder to interpret. One is the sandstone scale: the zero neutron reading is aligned with 2.65 g/cm3. Also, the neutron log may, or may not, be calibrated in sandstone units, reducing cross-over in sandstone by about two, or one, scale divisions, respectively.
If the two scales do not have the same amplitude (60 neutron porosity units corresponding to a range of 1 g/cm3), lithological interpretation should not be attempted from the overlay plot because log separations then become a function of porosity as well as lithology.
Lithological responses[edit]
Sandstone (Oil or Water Filled)[edit]
Clean quartz sandstones give the typical two-division neutron-density cross-over with density to the left of neutron (Figure 1). The addition of some clay (forming shaly sandstone) increases the neutron reading, reducing log crossover or even reversing it to create separation. Check natural gamma ray for evidence of increasing clay.
Heavier components such as mica increase the density, reducing log cross-over or even reversing it to create separation. Check spectral gamma ray to distinguish the following:
- Mica: potassium radiation only.
- Zircon (with other heavy minerals): thorium or uranium radiation.
- Siderite, pyrite, etc.: no increased radiation.
Use the shape of the neutron-density cross-over to provide depositional energy in the same way as an SP or gamma ray log (Figure 1). Thus, a “V” shape is a funnel (coarsening upward) and a “Λ” shape is a bell (fining upward).
Sandstone (Gas-Filled)[edit]
Compared to oil- or water-filled sandstone, the neutron log for a gas-filled sandstone reads as much as 10–15 porosity units too low, and the density log may read about 0.05 g/cm3 too low. Together these effects increase the log cross-over from two to about five scale divisions.
Sandstone (Air-Filled)[edit]
Nonhydrocarbon gas in sandstone can give neutron readings close to zero, depending on residual water and humidity in the pore space. Enormous log cross-over results.
Limestone[edit]
Clean limestone has no neutron-density separation (Figure 2). When the neutron drifts to higher values, expect the presence of clay. Check the natural gamma ray. In gas-filled limestone, expect cross-over like that described for sandstone, and use a Pe value of 5 to confirm limestone.
Dolomite[edit]
Characteristic four to six scale division separation with density to the right of neutron is relatively consistent in clean dolomite (Figure 2). Gas reduces or eliminates the separation; use a Pe value of 3 to confirm dolomite. Locally high natural gamma ray looks like clay, but if neutron-density separation is unchanged, it may be “hot” dolomite (especially in the Permian basin). Check uranium if spectral gamma ray is available.
Shale[edit]
Shale shows a log separation with neutron to the left of density, sometimes displaced by a large amount (Figure 1). At times the separation is only three or four scale divisions, which can resemble dolomite. To distinguish shale, check for the following:
- Apparent neutron porosity is too high for the area. Shale neutron readings are often between 30 and 50 porosity units.
- Caliper log shows washouts.
- Natural gamma ray is high; consistently high in beds where neutron is high. If spectral gamma ray is available, look for all radioactive elements elevated (contrast only uranium high in “hot” dolomite).
Coal[edit]
Neutron and density logs for coal both read similar very high apparent porosities (Figure 1c). Coals give prominent deflections that do not resemble anything but severe washouts. (Diatomite has a density of about 1.4 g/cm3 and a neutron measurement of about 60 porosity units, so crossover is at least seven scale divisions.)
Complex rock mixtures[edit]
Using neutron and density logs to resolve porosity and lithology allows only a “one-dimensional” view of lithology. Rock mixtures always create ambiguities for this simple quick-look interpretation. Local knowledge of rock types and mixtures to be expected and not to be expected may eliminate ambiguity (for example, do not look for dolomite and evaporites in a temperate, humid delta). Rock sample and mudlog data are invaluable. For complex rock mixtures, more input log data are needed, and computer-processed multidimensional crossplots must be used to determine lithology. In any case, confidence is always increased by using more input data.
See also[edit]
- Difficult lithologies
- Dipmeters
- Formation evaluation of naturally fractured reservoirs
- Basic open hole tools
- Basic tool table
- Introduction to wireline methods
- Determination of water resistivity
- Preprocessing of logging data
- Wireline formation testers
- Basic cased hole tools
- Standard interpretation
- Borehole imaging devices